Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer
- PMID: 20550524
- PMCID: PMC11159855
- DOI: 10.1111/j.1349-7006.2010.01624.x
Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer
Abstract
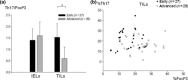
Although Th17 cells reportedly play critical roles in the development of autoimmunity and allergic reactions, information on Th17 cells in cancer-bearing hosts is still limited. In the present study, we investigated the distribution of Th17 cells in relation to regulatory T cells (Treg) in the tumor-infiltrating lymphocytes (TILs), regional lymph node lymphocytes, and peripheral blood lymphocytes of gastric cancer patients. Interleukin (IL)-17-producing CD4(+) cells as Th17 cells and CD4(+)CD25(+)FoxP3(+) cells as Treg were evaluated by flow cytometry and expressed as a percentage of the total CD4(+) cells, in addition to performing a Th1/Th2 balance assay. Moreover, immunohistochemical staining for IL-17 and FoxP3 were performed. In TILs from patients with early disease (n = 27), the frequency of Th17 cells was significantly higher than that in the normal gastric mucosa (23.7 ± 8.9 vs 4.5 ± 3.1%). In TILs from patients with advanced disease (n = 28), the frequency of Th17 cells was also significantly higher, but lower compared to early disease, than that in the normal gastric mucosa (15.1 ± 6.2 vs 4.0 ± 2.0%). This observation for Th17 cell-distribution was also confirmed by immunohistochemistry. When the ratio of Th17/Treg in TILs was evaluated in individual cases, it was more markedly increased in early than in advanced disease. In conclusion, the accumulation of Th17 cells as well as Treg in the tumor microenvironment of gastric cancer occurred in early disease and then the infiltration of Th17 cells gradually decreased according to the disease progression, in contrast to increased Treg.
© 2010 Japanese Cancer Association.
Figures





Similar articles
-
CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer.Int J Cancer. 2008 May 15;122(10):2286-93. doi: 10.1002/ijc.23392. Int J Cancer. 2008. PMID: 18224687
-
Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters.Oncol Rep. 2013 Sep;30(3):1215-22. doi: 10.3892/or.2013.2570. Epub 2013 Jun 27. Oncol Rep. 2013. PMID: 23807713
-
Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer.Oncol Rep. 2011 May;25(5):1271-7. doi: 10.3892/or.2011.1201. Epub 2011 Mar 1. Oncol Rep. 2011. PMID: 21369705
-
Regulatory T cell migration during an immune response.Trends Immunol. 2012 Apr;33(4):174-80. doi: 10.1016/j.it.2012.01.002. Epub 2012 Feb 2. Trends Immunol. 2012. PMID: 22305714 Free PMC article. Review.
-
Dendritic cell vaccination against ovarian cancer--tipping the Treg/TH17 balance to therapeutic advantage?Expert Opin Biol Ther. 2011 Apr;11(4):441-5. doi: 10.1517/14712598.2011.554812. Epub 2011 Jan 28. Expert Opin Biol Ther. 2011. PMID: 21271951 Free PMC article. Review.
Cited by
-
Gastric Cancer Stem Cells Effect on Th17/Treg Balance; A Bench to Beside Perspective.Front Oncol. 2019 Apr 5;9:226. doi: 10.3389/fonc.2019.00226. eCollection 2019. Front Oncol. 2019. PMID: 31024835 Free PMC article. Review.
-
Linking dysbiosis to precancerous stomach through inflammation: Deeper than and beyond imaging.Front Immunol. 2023 Mar 31;14:1134785. doi: 10.3389/fimmu.2023.1134785. eCollection 2023. Front Immunol. 2023. PMID: 37063848 Free PMC article. Review.
-
Th17 Cells in Protection from Tumor or Promotion of Tumor Progression.J Clin Cell Immunol. 2016 Jun;7(3):431. doi: 10.4172/2155-9899.1000431. Epub 2016 Jun 20. J Clin Cell Immunol. 2016. PMID: 27453801 Free PMC article.
-
VEGF165 attenuates the Th17/Treg imbalance that exists when transplanting allogeneic skeletal myoblasts to treat acute myocardial infarction.Inflamm Res. 2013 Jan;62(1):69-79. doi: 10.1007/s00011-012-0553-4. Epub 2012 Sep 21. Inflamm Res. 2013. PMID: 22996192
-
High Level of IL-10 in Cerebrospinal Fluid is Specific for Diagnosis of Primary Central Nervous System Lymphoma.Cancer Manag Res. 2020 Jul 24;12:6261-6268. doi: 10.2147/CMAR.S255482. eCollection 2020. Cancer Manag Res. 2020. PMID: 32801871 Free PMC article.
References
-
- Sakuramoto S, Sasako M, Yamaguchi T et al. , ACTS‐GC Group Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med 2007; 357: 1810–20. - PubMed
-
- MacDonald JS, Smalley SR, Benedetti J et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med, 2001; 345: 725–30. - PubMed
-
- Naito Y, Saito K, Shiiba K et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998; 58: 3491–4. - PubMed
-
- Bettelli E, Carrier Y, Gao W et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441: 235–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

