Pharmacokinetics and safety of subcutaneous immune globulin (human), 10% caprylate/chromatography purified in patients with primary immunodeficiency disease
- PMID: 20550549
- PMCID: PMC2962970
- DOI: 10.1111/j.1365-2249.2010.04195.x
Pharmacokinetics and safety of subcutaneous immune globulin (human), 10% caprylate/chromatography purified in patients with primary immunodeficiency disease
Abstract
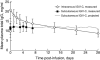
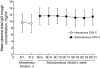
Subcutaneous administration of intravenous immunoglobulin G (IgG) preparations provides an additional level of patient convenience and more options for patients with poor venous access or a history of intravenous IgG reactions. An open-label, pharmacokinetic trial (n = 32) determined the non-inferiority of the subcutaneous versus intravenous route of 10% caprylate/chromatography purified human immune globulin intravenous (IGIV-C; Gamunex®) administration by comparing the steady-state area under the concentration-versus-time curve (AUC) of total plasma IgG in patients with primary immunodeficiency disease. Patients on stable IGIV-C received two intravenous infusions (administered 3 or 4 weeks apart). Seven to 10 days after the second intravenous infusion, all patients switched to a weekly infusion of subcutaneous IGIV-C, with the dose equal to 137% of the previous weekly equivalent intravenous dose, for up to 24 weeks. Samples for pharmacokinetic analysis were collected during steady state for intravenous and subcutaneous IGIV-C treatments. The AUC(0-) τ geometric least-squares mean ratio was 0·89 (90% confidence interval, 0·86-0·92) and met the criteria for non-inferiority. The overall mean steady-state trough concentration of plasma total IgG with subcutaneous IGIV-C was 11·4 mg/ml, 18·8% higher than intravenous IGIV-C (9·6 mg/ml). Subcutaneous IGIV-C was safe and well tolerated. Subcutaneous IGIV-C infusion-site reactions were generally mild/moderate and the incidence decreased over time. No serious bacterial infections were reported. Weekly subcutaneous IGIV-C infusion using 137% of the weekly equivalent intravenous immunoglobulin dose provides an AUC comparable to intravenous administration, thus allowing patients to maintain the same IgG preparation/formulation if switching between intravenous and subcutaneous infusions.
© 2010 British Society for Immunology.
Figures

Similar articles
-
Pharmacokinetics, Safety, and Tolerability of Subcutaneous Immune Globulin Injection (Human), 10 % Caprylate/Chromatography Purified (GAMUNEX®-C) in Pediatric Patients with Primary Immunodeficiency Disease.J Clin Immunol. 2016 Aug;36(6):600-9. doi: 10.1007/s10875-016-0311-4. Epub 2016 Jun 25. J Clin Immunol. 2016. PMID: 27342758 Clinical Trial.
-
Immune globulin subcutaneous, human - klhw 20% for primary humoral immunodeficiency: an open-label, Phase III study.Immunotherapy. 2019 Nov;11(16):1371-1386. doi: 10.2217/imt-2019-0159. Epub 2019 Oct 17. Immunotherapy. 2019. PMID: 31621458 Clinical Trial.
-
A multi-centre study of efficacy and safety of Intratect®, a novel intravenous immunoglobulin preparation.Clin Exp Immunol. 2010 Sep;161(3):512-7. doi: 10.1111/j.1365-2249.2010.04187.x. Clin Exp Immunol. 2010. PMID: 20550545 Free PMC article. Clinical Trial.
-
Immune globulin (human) 10 % liquid: a review of its use in primary immunodeficiency disorders.BioDrugs. 2013 Aug;27(4):393-400. doi: 10.1007/s40259-013-0044-3. BioDrugs. 2013. PMID: 23703447 Review.
-
Emerging Paradigm of Primary Immunodeficiency Disease: Individualizing Immunoglobulin Dose and Delivery to Enhance Outcomes.J Clin Immunol. 2017 Feb;37(2):190-196. doi: 10.1007/s10875-014-9990-x. Epub 2014 Jan 30. J Clin Immunol. 2017. PMID: 24477950 Review.
Cited by
-
Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review.Drugs. 2013 Aug;73(12):1307-19. doi: 10.1007/s40265-013-0094-3. Drugs. 2013. PMID: 23861187 Review.
-
Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy.Clin Exp Immunol. 2012 Aug;169(2):172-81. doi: 10.1111/j.1365-2249.2012.04594.x. Clin Exp Immunol. 2012. PMID: 22774992 Free PMC article.
-
British Society for Immunology and United Kingdom Primary Immunodeficiency Network (UKPIN) consensus guideline for the management of immunoglobulin replacement therapy.Clin Exp Immunol. 2022 Oct 21;210(1):1-13. doi: 10.1093/cei/uxac070. Clin Exp Immunol. 2022. PMID: 35924867 Free PMC article.
-
Broadening the translational immunology landscape.Clin Exp Immunol. 2012 Dec;170(3):249-53. doi: 10.1111/j.1365-2249.2012.04671.x. Clin Exp Immunol. 2012. PMID: 23121665 Free PMC article.
-
Steady-State Serum IgG Trough Levels Are Adequate for Pharmacokinetic Assessment in Patients with Immunodeficiencies Receiving Subcutaneous Immune Globulin.J Clin Immunol. 2021 Aug;41(6):1331-1338. doi: 10.1007/s10875-021-00990-z. Epub 2021 May 26. J Clin Immunol. 2021. PMID: 34036490 Free PMC article.
References
-
- Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117(Suppl.):S525–53. - PubMed
-
- Bonilla FA. Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunol Allergy Clin North Am. 2008;28:803–19. ix. - PubMed
-
- Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M, the Subcutaneous IgG Study Group Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–73. - PubMed
-
- Gardulf A, Nicolay U, Asensio O, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies – a prospective, multi-national study. J Clin Immunol. 2006;26:177–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

