Design considerations for a multicenter randomized controlled trial of early surgery for mesial temporal lobe epilepsy
- PMID: 20550556
- PMCID: PMC2941700
- DOI: 10.1111/j.1528-1167.2010.02641.x
Design considerations for a multicenter randomized controlled trial of early surgery for mesial temporal lobe epilepsy
Abstract
Purpose: To describe the trial design for the multicenter Early Randomized Surgical Epilepsy Trial (ERSET). Patients with pharmacoresistant epilepsy are generally referred for surgical treatment an average of two decades after onset of seizures, often too late to avoid irreversible disability. ERSET was designed to assess the safety and efficacy of early surgical intervention compared to continued pharmacotherapy.
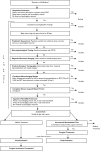
Methods: ERSET is a randomized controlled, parallel group clinical trial with blinded outcome adjudication. Participants are patients with mesial temporal lobe epilepsy (MTLE) older than the age of 12 who have had pharmacoresistant seizures for not >2 years and are determined by detailed evaluation to be surgical candidates prior to randomization. The primary outcome measure is seizure freedom in the second year of a 2-year follow-up period. Health-related quality of life (HRQOL), neurocognitive function, ancillary outcomes, and adverse events were also measured.
Results: Significant methodologic problems addressed by the study design included the following: recruitment of participants early in the course of epilepsy; establishment of operational definitions for "pharmacoresistant" and "early"; and standardization of diagnostic testing, medical treatment, and surgical interventions across multiple centers.
Discussion: Rigorous trial designs to assess surgical interventions in epilepsy are necessary to provide evidence to guide treatment. This article is the first of a series; trial results will be reported in subsequent publications.
Wiley Periodicals, Inc. © 2010 International League Against Epilepsy.
References
-
- Achenbach T, Edelbrock C. Manual for the Child Behavior Checklist and 4–18 and 1991 Profile. Department of Psychiatry, University of Vermont; 1991.
-
- Bauer J, Buchmüller L, Reuber M, Burr W. Which patients become seizure free with antiepileptic drugs? An observational study in 821 patients with epilepsy. Acta Neurologica Scandinavica. 2008;117:55–59. - PubMed
-
- Benedict RHB. Brief Visuo-spatial Memory Test-Revised. Psychological Assessment Resources, Inc.; Odessa, FL: 1997.
-
- Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, Bazil C, Pacia SV, Spencer SS. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60:186–190. - PubMed
-
- Berg AT, Shinnar S, Levy SR, Testa FM. Newly-diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999;40:445–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources