A model of fibroblast motility on substrates with different rigidities
- PMID: 20550891
- PMCID: PMC2884250
- DOI: 10.1016/j.bpj.2010.03.026
A model of fibroblast motility on substrates with different rigidities
Erratum in
- Biophys J. 2011 Feb 2;100(3):795
-
Correction.Biophys J. 2011 Feb 2;100(3):795. doi: 10.1016/j.bpj.2011.01.015. Epub 2011 Feb 1. Biophys J. 2011. PMID: 30021262 Free PMC article. No abstract available.
Abstract
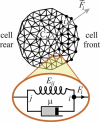
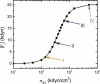
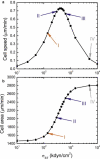
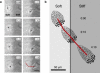
To function efficiently in the body, the biological cells must have the ability to sense the external environment. Mechanosensitivity toward the extracellular matrix was identified as one of the sensing mechanisms affecting cell behavior. It was shown experimentally that a fibroblast cell prefers locomoting over the stiffer substrate when given a choice between a softer and a stiffer substrate. In this article, we develop a discrete model of fibroblast motility with substrate-rigidity sensing. Our model allows us to understand the interplay between the cell-substrate sensing and the cell biomechanics. The model cell exhibits experimentally observed substrate rigidity sensing, which allows us to gain additional insights into the cell mechanosensitivity.
(c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Discher D.E., Janmey P., Wang Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. - PubMed
-
- Chicurel M. Cell biology. Cell migration research is on the move. Science. 2002;295:606–609. - PubMed
-
- Bray D. Garland Publishing; New York: 2001. Cell Movements: From Molecules to Motility.
-
- Izzard C.S., Lochner L.R. Formation of cell-to-substrate contacts during fibroblast motility: an interference-reflection study. J. Cell Sci. 1980;80:81–116. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

