Down-state model of the voltage-sensing domain of a potassium channel
- PMID: 20550898
- PMCID: PMC2884232
- DOI: 10.1016/j.bpj.2010.03.031
Down-state model of the voltage-sensing domain of a potassium channel
Abstract
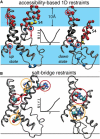
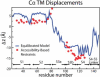
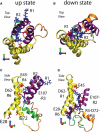
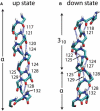
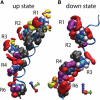
Voltage-sensing domains (VSDs) of voltage-gated potassium (Kv) channels undergo a series of conformational changes upon membrane depolarization, from a down state when the channel is at rest to an up state, all of which lead to the opening of the channel pore. The crystal structures reported to date reveal the pore in an open state and the VSDs in an up state. To gain insights into the structure of the down state, we used a set of experiment-based restraints to generate a model of the down state of the KvAP VSD using molecular-dynamics simulations of the VSD in a lipid bilayer in excess water. The equilibrated VSD configuration is consistent with the biotin-avidin accessibility and internal salt-bridge data used to generate it, and with additional biotin-avidin accessibility data. In the model, both the S3b and S4 segments are displaced approximately 10 A toward the intracellular side with respect to the up-state configuration, but they do not move as a rigid body. Arginine side chains that carry the majority of the gating charge also make large excursions between the up and down states. In both states, arginines interact with water and participate in salt bridges with acidic residues and lipid phosphate groups. An important feature that emerges from the down-state model is that the N-terminal half of the S4 segment adopts a 3(10)-helical conformation, which appears to be necessary to satisfy a complex salt-bridge network.
(c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Hille B. Sinauer Associates, Inc.; Sunderland, MA: 2001. Ion Channels of Excitable Membranes.
-
- MacKinnon R., Aldrich R.W., Lee A.W. Functional stoichiometry of Shaker potassium channel inactivation. Science. 1993;262:757–759. - PubMed
-
- Jiang Y.X., Lee A., MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. - PubMed
-
- Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 2000;80:555–592. - PubMed
-
- Tombola F., Pathak M.M., Isacoff E.Y. How does voltage open an ion channel? Annu. Rev. Cell Dev. Biol. 2006;22:23–52. - PubMed

