Millisecond timescale dynamics of human liver fatty acid binding protein: testing of its relevance to the ligand entry process
- PMID: 20550918
- PMCID: PMC2884256
- DOI: 10.1016/j.bpj.2010.03.047
Millisecond timescale dynamics of human liver fatty acid binding protein: testing of its relevance to the ligand entry process
Abstract
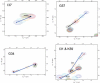
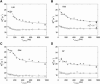
For over a decade, scientists have been attempting to know more about the conformational dynamics of fatty acid binding proteins (FABPs), to answer the puzzling question of how ligands could access the internalized binding site(s). Conformational exchange of FABPs on the microsecond to millisecond timescales has been found in many FABPs and offers an important hypothesis for the ligand entry mechanism. Despite the potential significance, the validity of this hypothesis has not been verified yet. In this study, the slow dynamics of human liver fatty acid binding protein (hLFABP) that was shown previously to be highly flexible on millisecond timescales was quantitatively characterized in detail. In addition, the interaction between hLFABP and 1,8-ANS was studied using NMR spectroscopy, and the kinetic rate of ANS association to hLFABP was measured. We believe the current result excludes the possibility that the intrinsic millisecond dynamics of hLFABP represents a critical conformational reorganization process required for ligand entry, but implies that it may represent the exchange between the apo-state and a state resembling the singly-bound conformation. Furthermore, we suggest these results show that the ligand-entry related functional dynamics could occur on the microsecond/submicrosecond timescales, highly encouraging future computational studies on this topic.
(c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Storch J., Corsico B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 2008;28:73–95. - PubMed
-
- Thompson J., Reese-Wagoner A., Banaszak L. Liver fatty acid binding protein: species variation and the accommodation of different ligands. Biochim. Biophys. Acta. 1999;1441:117–130. - PubMed
-
- Xu Z., Bernlohr D.A., Banaszak L.J. The adipocyte lipid-binding protein at 1.6-A resolution. Crystal structures of the apoprotein and with bound saturated and unsaturated fatty acids. J. Biol. Chem. 1993;268:7874–7884. - PubMed
-
- Young A.C., Scapin G., Sacchettini J.C. Structural studies on human muscle fatty acid binding protein at 1.4 A resolution: binding interactions with three C18 fatty acids. Structure. 1994;2:523–534. - PubMed
-
- Lassen D., Lücke C., Rüterjans H. Three-dimensional structure of bovine heart fatty-acid-binding protein with bound palmitic acid, determined by multidimensional NMR spectroscopy. Eur. J. Biochem. 1995;230:266–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

