A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production
- PMID: 20550936
- PMCID: PMC2910436
- DOI: 10.1016/j.cell.2010.04.040
A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production
Abstract
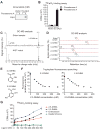
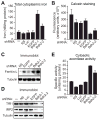
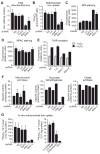
Intracellular iron homeostasis is critical for survival and proliferation. Lipocalin 24p3 is an iron-trafficking protein that binds iron through association with a bacterial siderophore, such as enterobactin, or a postulated mammalian siderophore. Here, we show that the iron-binding moiety of the 24p3-associated mammalian siderophore is 2,5-dihydroxybenzoic acid (2,5-DHBA), which is similar to 2,3-DHBA, the iron-binding component of enterobactin. We find that the murine enzyme responsible for 2,5-DHBA synthesis, BDH2, is the homolog of bacterial EntA, which catalyzes 2,3-DHBA production during enterobactin biosynthesis. RNA interference-mediated knockdown of BDH2 results in siderophore depletion. Mammalian cells lacking the siderophore accumulate abnormally high amounts of cytoplasmic iron, resulting in elevated levels of reactive oxygen species, whereas the mitochondria are iron deficient. Siderophore-depleted mammalian cells and zebrafish embryos fail to synthesize heme, an iron-dependent mitochondrial process. Our results reveal features of intracellular iron homeostasis that are conserved from bacteria through humans.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Akerstrom B, Flower DR, Salier JP. Lipocalins: unity in diversity. Biochim Biophys Acta. 2000;1482:1–8. - PubMed
-
- Blat D, Weiner L, Youdim MB, Fridkin M. A novel iron-chelating derivative of the neuroprotective peptide NAPVSIPQ shows superior antioxidant and antineurodegenerative capabilities. J Med Chem. 2008;51:126–134. - PubMed
-
- Breuer W, Shvartsman M, Cabantchik ZI. Intracellular labile iron. Int J Biochem Cell Biol. 2008;40:350–354. - PubMed
-
- Brownlie A, Donovan A, Pratt SJ, Paw BH, Oates AC, Brugnara C, Witkowska HE, Sassa S, Zon LI. Positional cloning of the zebrafish sauternes gene: a model for congenital sideroblastic anaemia. Nat Genet. 1998;20:244–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

