The interaction between Myc and Miz1 is required to antagonize TGFbeta-dependent autocrine signaling during lymphoma formation and maintenance
- PMID: 20551174
- PMCID: PMC2885663
- DOI: 10.1101/gad.585710
The interaction between Myc and Miz1 is required to antagonize TGFbeta-dependent autocrine signaling during lymphoma formation and maintenance
Abstract
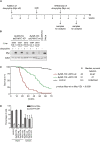
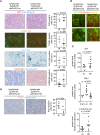
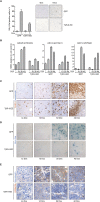
The Myc protein suppresses the transcription of several cyclin-dependent kinase inhibitors (CKIs) via binding to Miz1; whether this interaction is important for Myc's ability to induce or maintain tumorigenesis is not known. Here we show that the oncogenic potential of a point mutant of Myc (MycV394D) that is selectively deficient in binding to Miz1 is greatly attenuated. Binding of Myc to Miz1 is continuously required to repress CKI expression and inhibit accumulation of trimethylated histone H3 at Lys 9 (H3K9triMe), a hallmark of cellular senescence, in T-cell lymphomas. Lymphomas that arise express high amounts of transforming growth factor beta-2 (TGFbeta-2) and TGFbeta-3. Upon Myc suppression, TGFbeta signaling is required to induce CKI expression and cellular senescence and suppress tumor recurrence. Binding of Myc to Miz1 is required to antagonize growth suppression and induction of senescence by TGFbeta. We demonstrate that, since lymphomas express high levels of TGFbeta, they are poised to elicit an autocrine program of senescence upon Myc inactivation, demonstrating that TGFbeta is a key factor that establishes oncogene addiction of T-cell lymphomas.
Figures





Comment in
-
Tumorigenesis: Might as well face it, you're addicted to MYC.Nat Rev Cancer. 2010 Aug;10(8):532-3. doi: 10.1038/nrc2908. Nat Rev Cancer. 2010. PMID: 20677353 No abstract available.
References
-
- Benassi B, Fanciulli M, Fiorentino F, Porrello A, Chiorino G, Loda M, Zupi G, Biroccio A 2006. c-Myc phosphorylation is required for cellular response to oxidative stress. Mol Cell 21: 509–519 - PubMed
-
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA 2005. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436: 660–665 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
