Cdx2 regulates endo-lysosomal function and epithelial cell polarity
- PMID: 20551175
- PMCID: PMC2885664
- DOI: 10.1101/gad.1921510
Cdx2 regulates endo-lysosomal function and epithelial cell polarity
Abstract
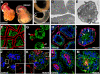
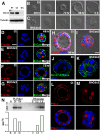
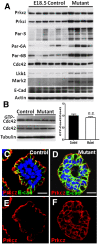
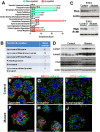
In contrast to our significant understanding of signaling cascades that determine cell polarity in lower eukaryotic or immortalized cells, little is known about the transcriptional program that governs mammalian epithelial polarization in vivo. Here we show, using conditional gene ablation and three-dimensional tissue culture, that the homeobox transcription factor Cdx2 controls apical-basolateral polarity in mouse enterocytes and human colonic epithelial cells. Cdx2 regulates a comprehensive gene network involved in endo-lysosomal maturation and protein transport. In the absence of Cdx2, defective protein trafficking impairs apical-basal transport and induces ectopic lumen formation. These defects are partially recapitulated by suppression of key apical transport components, Rab11a and Kif3b, which are regulated by Cdx2. Furthermore, Cdx2 deficiency affects components that control the organization of microvillus actin cytoskeleton, leading to severe microvillus atrophy. These results demonstrate that Cdx2 regulates epithelial cell polarity and morphogenesis through control of apical protein transport and endo-lysosomal function.
Figures

Similar articles
-
Cell polarity regulator PARD6B is essential for trophectoderm formation in the preimplantation mouse embryo.Biol Reprod. 2010 Sep;83(3):347-58. doi: 10.1095/biolreprod.110.084400. Epub 2010 May 26. Biol Reprod. 2010. PMID: 20505164 Free PMC article.
-
The intestine-specific homeobox gene Cdx2 induces expression of the basic helix-loop-helix transcription factor Math1.Differentiation. 2006 Jul;74(6):313-21. doi: 10.1111/j.1432-0436.2006.00074.x. Differentiation. 2006. PMID: 16831200
-
Rab11a regulates syntaxin 3 localization and microvillus assembly in enterocytes.J Cell Sci. 2015 Apr 15;128(8):1617-26. doi: 10.1242/jcs.163303. Epub 2015 Feb 11. J Cell Sci. 2015. PMID: 25673875 Free PMC article.
-
Transcription factor AP-2γ induces early Cdx2 expression and represses HIPPO signaling to specify the trophectoderm lineage.Development. 2015 May 1;142(9):1606-15. doi: 10.1242/dev.120238. Epub 2015 Apr 9. Development. 2015. PMID: 25858457 Free PMC article.
-
Intestinal epithelial cell polarity defects in disease: lessons from microvillus inclusion disease.Dis Model Mech. 2018 Feb 13;11(2):dmm031088. doi: 10.1242/dmm.031088. Dis Model Mech. 2018. PMID: 29590640 Free PMC article. Review.
Cited by
-
Rab8a vesicles regulate Wnt ligand delivery and Paneth cell maturation at the intestinal stem cell niche.Development. 2015 Jun 15;142(12):2147-62. doi: 10.1242/dev.121046. Epub 2015 May 26. Development. 2015. PMID: 26015543 Free PMC article.
-
Pluripotency of periodontal ligament and dental pulp cells is induced by intercellular communication via the CDX2/Oct-4/Sox2 pathway.Ann Transl Med. 2022 Aug;10(16):868. doi: 10.21037/atm-22-3492. Ann Transl Med. 2022. PMID: 36111038 Free PMC article.
-
Ephrin-A1 inhibits NSCLC tumor growth via induction of Cdx-2 a tumor suppressor gene.BMC Cancer. 2012 Jul 23;12:309. doi: 10.1186/1471-2407-12-309. BMC Cancer. 2012. PMID: 22824143 Free PMC article.
-
Recycling Endosomes in Mature Epithelia Restrain Tumorigenic Signaling.Cancer Res. 2019 Aug 15;79(16):4099-4112. doi: 10.1158/0008-5472.CAN-18-4075. Epub 2019 Jun 25. Cancer Res. 2019. PMID: 31239271 Free PMC article.
-
In vitro patterning of pluripotent stem cell-derived intestine recapitulates in vivo human development.Development. 2017 Mar 15;144(6):1045-1055. doi: 10.1242/dev.138453. Epub 2016 Dec 7. Development. 2017. PMID: 27927684 Free PMC article.
References
-
- Ameen N, Apodaca G 2007. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998–1006 - PubMed
-
- Baricault L, Garcia M, Cibert C, Sapin C, Geraud G, Codogno P, Trugnan G 1993. Forskolin blocks the apical expression of dipeptidyl peptidase IV in Caco-2 cells and induces its retention in lamp-1-containing vesicles. Exp Cell Res 209: 277–287 - PubMed
-
- Cramm-Behrens CI, Dienst M, Jacob R 2008. Apical cargo traverses endosomal compartments on the passage to the cell surface. Traffic 9: 2206–2220 - PubMed
-
- Debnath J, Muthuswamy SK, Brugge JS 2003. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30: 256–268 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
