A novel phosphatase cascade regulates differentiation in Trypanosoma brucei via a glycosomal signaling pathway
- PMID: 20551176
- PMCID: PMC2885665
- DOI: 10.1101/gad.570310
A novel phosphatase cascade regulates differentiation in Trypanosoma brucei via a glycosomal signaling pathway
Abstract
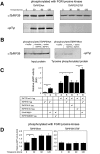
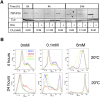
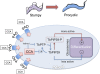
In the mammalian bloodstream, the sleeping sickness parasite Trypanosoma brucei is held poised for transmission by the activity of a tyrosine phosphatase, TbPTP1. This prevents differentiation of the transmissible "stumpy forms" until entry into the tsetse fly, whereupon TbPTP1 is inactivated and major changes in parasite physiology are initiated to allow colonization of the arthropod vector. Using a substrate-trapping approach, we identified the downstream step in this developmental signaling pathway as a DxDxT phosphatase, TbPIP39, which is activated upon tyrosine phosphorylation, and hence is negatively regulated by TbPTP1. In vitro, TbPIP39 promotes the activity of TbPTP1, thereby reinforcing its own repression, this being alleviated by the trypanosome differentiation triggers citrate and cis-aconitate, generating a potentially bistable regulatory switch. Supporting a role in signal transduction, TbPIP39 becomes rapidly tyrosine-phosphorylated during differentiation, and RNAi-mediated transcript ablation in stumpy forms inhibits parasite development. Interestingly, TbPIP39 localizes in glycosomes, peroxisome-like organelles that compartmentalize the trypanosome glycolytic reactions among other enzymatic activities. Our results invoke a phosphatase signaling cascade in which the developmental signal is trafficked to a unique metabolic organelle in the parasite: the glycosome. This is the first characterized environmental signaling pathway targeted directly to a peroxisome-like organelle in any eukaryotic cell.
Figures



Similar articles
-
Positional Dynamics and Glycosomal Recruitment of Developmental Regulators during Trypanosome Differentiation.mBio. 2019 Jul 9;10(4):e00875-19. doi: 10.1128/mBio.00875-19. mBio. 2019. PMID: 31289175 Free PMC article.
-
Protein tyrosine phosphatase TbPTP1: A molecular switch controlling life cycle differentiation in trypanosomes.J Cell Biol. 2006 Oct 23;175(2):293-303. doi: 10.1083/jcb.200605090. Epub 2006 Oct 16. J Cell Biol. 2006. PMID: 17043136 Free PMC article.
-
The Trypanosoma brucei life cycle switch TbPTP1 is structurally conserved and dephosphorylates the nucleolar protein NOPP44/46.J Biol Chem. 2010 Jul 16;285(29):22075-81. doi: 10.1074/jbc.M110.108860. Epub 2010 May 5. J Biol Chem. 2010. PMID: 20444707 Free PMC article.
-
Glycosomes: A comprehensive view of their metabolic roles in T. brucei.Int J Biochem Cell Biol. 2017 Apr;85:85-90. doi: 10.1016/j.biocel.2017.01.015. Epub 2017 Feb 6. Int J Biochem Cell Biol. 2017. PMID: 28179189 Review.
-
Biogenesis, maintenance and dynamics of glycosomes in trypanosomatid parasites.Biochim Biophys Acta. 2016 May;1863(5):1038-48. doi: 10.1016/j.bbamcr.2015.09.015. Epub 2015 Sep 16. Biochim Biophys Acta. 2016. PMID: 26384872 Review.
Cited by
-
Allostery in Protein Tyrosine Phosphatases is Enabled by Divergent Dynamics.J Chem Inf Model. 2024 Feb 26;64(4):1331-1346. doi: 10.1021/acs.jcim.3c01615. Epub 2024 Feb 12. J Chem Inf Model. 2024. PMID: 38346324 Free PMC article.
-
In silico characterization of an atypical MAPK phosphatase of Plasmodium falciparum as a suitable target for drug discovery.Chem Biol Drug Des. 2014 Aug;84(2):158-68. doi: 10.1111/cbdd.12315. Epub 2014 May 12. Chem Biol Drug Des. 2014. PMID: 24605883 Free PMC article.
-
Suramin exposure alters cellular metabolism and mitochondrial energy production in African trypanosomes.J Biol Chem. 2020 Jun 12;295(24):8331-8347. doi: 10.1074/jbc.RA120.012355. Epub 2020 Apr 30. J Biol Chem. 2020. PMID: 32354742 Free PMC article.
-
Global proteomic analysis in trypanosomes reveals unique proteins and conserved cellular processes impacted by arginine methylation.J Proteomics. 2013 Oct 8;91:210-25. doi: 10.1016/j.jprot.2013.07.010. Epub 2013 Jul 19. J Proteomics. 2013. PMID: 23872088 Free PMC article.
-
Redistribution of FLAgellar Member 8 during the trypanosome life cycle: Consequences for cell fate prediction.Cell Microbiol. 2021 Sep;23(9):e13347. doi: 10.1111/cmi.13347. Epub 2021 May 14. Cell Microbiol. 2021. PMID: 33896083 Free PMC article.
References
-
- Albert MA, Haanstra JR, Hannaert V, Van Roy J, Opperdoes FR, Bakker BM, Michels PA 2005. Experimental and in silico analyses of glycolytic flux control in bloodstream form Trypanosoma brucei. J Biol Chem 280: 28306–28315 - PubMed
-
- Barrett MP, Burchmore RJ, Stich A, Lazzari JO, Frasch AC, Cazzulo JJ, Krishna S 2003. The trypanosomiases. Lancet 362: 1469–1480 - PubMed
-
- Bastin P, Bagherzadeh Z, Matthews KR, Gull K 1996. A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Mol Biochem Parasitol 77: 235–239 - PubMed
-
- Biebinger S, Wirtz LE, Lorenz P, Clayton C 1997. Vectors for inducible expression of toxic gene products in bloodstream and procyclic Trypanosoma brucei. Mol Biochem Parasitol 85: 99–112 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
