GABA(A) receptor alpha1 subunit mutation A322D associated with autosomal dominant juvenile myoclonic epilepsy reduces the expression and alters the composition of wild type GABA(A) receptors
- PMID: 20551311
- PMCID: PMC2924069
- DOI: 10.1074/jbc.M110.142299
GABA(A) receptor alpha1 subunit mutation A322D associated with autosomal dominant juvenile myoclonic epilepsy reduces the expression and alters the composition of wild type GABA(A) receptors
Abstract
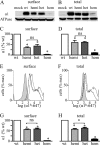
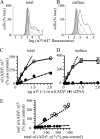
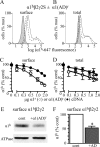
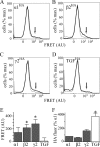
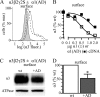
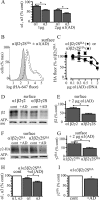
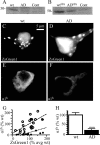
A GABA(A) receptor (GABA(A)R) alpha1 subunit mutation, A322D (AD), causes an autosomal dominant form of juvenile myoclonic epilepsy (ADJME). Previous studies demonstrated that the mutation caused alpha1(AD) subunit misfolding and rapid degradation, reducing its total and surface expression substantially. Here, we determined the effects of the residual alpha1(AD) subunit expression on wild type GABA(A)R expression to determine whether the AD mutation conferred a dominant negative effect. We found that although the alpha1(AD) subunit did not substitute for wild type alpha1 subunits on the cell surface, it reduced the surface expression of alpha1beta2gamma2 and alpha3beta2gamma2 receptors by associating with the wild type subunits within the endoplasmic reticulum and preventing them from trafficking to the cell surface. The alpha1(AD) subunit reduced surface expression of alpha3beta2gamma2 receptors by a greater amount than alpha1beta2gamma2 receptors, thus altering cell surface GABA(A)R composition. When transfected into cultured cortical neurons, the alpha1(AD) subunit altered the time course of miniature inhibitory postsynaptic current kinetics and reduced miniature inhibitory postsynaptic current amplitudes. These findings demonstrated that, in addition to causing a heterozygous loss of function of alpha1(AD) subunits, this epilepsy mutation also elicited a modest dominant negative effect that likely shapes the epilepsy phenotype.
Figures
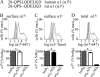
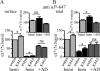

Similar articles
-
The developmental evolution of the seizure phenotype and cortical inhibition in mouse models of juvenile myoclonic epilepsy.Neurobiol Dis. 2015 Oct;82:164-175. doi: 10.1016/j.nbd.2015.05.016. Epub 2015 Jun 6. Neurobiol Dis. 2015. PMID: 26054439 Free PMC article.
-
The juvenile myoclonic epilepsy GABA(A) receptor alpha1 subunit mutation A322D produces asymmetrical, subunit position-dependent reduction of heterozygous receptor currents and alpha1 subunit protein expression.J Neurosci. 2004 Jun 16;24(24):5570-8. doi: 10.1523/JNEUROSCI.1301-04.2004. J Neurosci. 2004. PMID: 15201329 Free PMC article.
-
The GABAA receptor alpha1 subunit epilepsy mutation A322D inhibits transmembrane helix formation and causes proteasomal degradation.Proc Natl Acad Sci U S A. 2007 Aug 7;104(32):12999-3004. doi: 10.1073/pnas.0700163104. Epub 2007 Aug 1. Proc Natl Acad Sci U S A. 2007. PMID: 17670950 Free PMC article.
-
Autosomal dominant juvenile myoclonic epilepsy and GABRA1.Adv Neurol. 2005;95:255-63. Adv Neurol. 2005. PMID: 15508928 Review. No abstract available.
-
Molecular Pathogenic Basis for GABRG2 Mutations Associated With a Spectrum of Epilepsy Syndromes, From Generalized Absence Epilepsy to Dravet Syndrome.JAMA Neurol. 2016 Aug 1;73(8):1009-16. doi: 10.1001/jamaneurol.2016.0449. JAMA Neurol. 2016. PMID: 27367160 Free PMC article. Review.
Cited by
-
Genetic Animal Models of Idiopathic Generalized Epilepsies: What Can We Learn from Them?Biomedicines. 2025 May 26;13(6):1301. doi: 10.3390/biomedicines13061301. Biomedicines. 2025. PMID: 40564020 Free PMC article. Review.
-
The GABRA6 mutation, R46W, associated with childhood absence epilepsy, alters 6β22 and 6β2 GABA(A) receptor channel gating and expression.J Physiol. 2011 Dec 1;589(Pt 23):5857-78. doi: 10.1113/jphysiol.2011.218883. Epub 2011 Sep 19. J Physiol. 2011. PMID: 21930603 Free PMC article.
-
Dodecyl maltopyranoside enabled purification of active human GABA type A receptors for deep and direct proteomic sequencing.Mol Cell Proteomics. 2015 Mar;14(3):724-38. doi: 10.1074/mcp.M114.042556. Epub 2014 Dec 3. Mol Cell Proteomics. 2015. PMID: 25473089 Free PMC article.
-
The effect of HSP-causing mutations in SPG3A and NIPA1 on the assembly, trafficking, and interaction between atlastin-1 and NIPA1.Mol Cell Neurosci. 2011 Jan;46(1):122-35. doi: 10.1016/j.mcn.2010.08.012. Epub 2010 Sep 21. Mol Cell Neurosci. 2011. PMID: 20816793 Free PMC article.
-
Single-cell genetic expression of mutant GABAA receptors causing Human genetic epilepsy alters dendritic spine and GABAergic bouton formation in a mutation-specific manner.Front Cell Neurosci. 2014 Oct 14;8:317. doi: 10.3389/fncel.2014.00317. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25352779 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

