Mutual rescues between two dominant negative mutations in cardiac troponin I and cardiac troponin T
- PMID: 20551314
- PMCID: PMC2934648
- DOI: 10.1074/jbc.M110.137844
Mutual rescues between two dominant negative mutations in cardiac troponin I and cardiac troponin T
Abstract
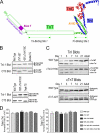
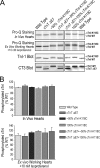
Troponin T (TnT) and troponin I (TnI) are two evolutionarily and functionally linked subunits of the troponin complex that regulates striated muscle contraction. We previously reported a single amino acid substitution in the highly conserved TnT-binding helix of cardiac TnI (cTnI) in wild turkey hearts in concurrence with an abnormally spliced myopathic cardiac TnT (cTnT) (Biesiadecki, B. J., Schneider, K. L., Yu, Z. B., Chong, S. M., and Jin, J. P. (2004) J. Biol. Chem. 279, 13825-13832). To investigate the functional effect of this cTnI mutation and its potential value in compensating for the cTnT abnormality, we developed transgenic mice expressing the mutant cTnI (K118C) in the heart with or without the deletion of the endogenous cTnI gene to mimic the homozygote and heterozygote of wild turkeys. Double and triple transgenic mice were created by crossing the cTnI-K118C lines with transgenic mice overexpressing the myopathic cTnT (exon 7 deletion). Functional studies of ex vivo working hearts found that cTnI-K118C alone had a dominantly negative effect on diastolic function and blunted the inotropic responses of cardiac muscle to beta-adrenergic stimuli without abolishing the protein kinase A-dependent phosphorylation of cTnI. When co-expressed with the cTnT mutation, cTnI-K118C corrected the significant depression of systolic function caused by cTnT exon 7 deletion, and the co-existence of exon 7-deleted cTnT minimized the diastolic abnormality of cTnI-K118C. Characterization of this naturally selected pair of mutually rescuing mutations demonstrated that TnI-TnT interaction is a critical link in the Ca(2+) signaling and beta-adrenergic regulation in cardiac muscle, suggesting a potential target for the treatment of troponin cardiomyopathies and heart failure.
Figures





Similar articles
-
Distinct conformational and functional effects of two adjacent pathogenic mutations in cardiac troponin I at the interface with troponin T.FEBS Open Bio. 2015 Jan 13;5:64-75. doi: 10.1016/j.fob.2015.01.001. eCollection 2015. FEBS Open Bio. 2015. PMID: 25685665 Free PMC article.
-
A dominantly negative mutation in cardiac troponin I at the interface with troponin T causes early remodeling in ventricular cardiomyocytes.Am J Physiol Cell Physiol. 2014 Aug 15;307(4):C338-48. doi: 10.1152/ajpcell.00053.2014. Epub 2014 Jun 4. Am J Physiol Cell Physiol. 2014. PMID: 24898585 Free PMC article.
-
NH2-terminal truncations of cardiac troponin I and cardiac troponin T produce distinct effects on contractility and calcium homeostasis in adult cardiomyocytes.Am J Physiol Cell Physiol. 2015 Mar 1;308(5):C397-404. doi: 10.1152/ajpcell.00358.2014. Epub 2014 Dec 17. Am J Physiol Cell Physiol. 2015. PMID: 25518962 Free PMC article.
-
The miscommunicative cardiac cell: when good proteins go bad.Ann N Y Acad Sci. 2005 Jun;1047:30-7. doi: 10.1196/annals.1341.003. Ann N Y Acad Sci. 2005. PMID: 16093482 Review.
-
Cardiac troponin I and troponin T: are enzymes still relevant as cardiac markers?Clin Chim Acta. 1997 Jan 3;257(1):99-115. doi: 10.1016/s0009-8981(96)06436-4. Clin Chim Acta. 1997. PMID: 9028628 Review.
Cited by
-
Distinct conformational and functional effects of two adjacent pathogenic mutations in cardiac troponin I at the interface with troponin T.FEBS Open Bio. 2015 Jan 13;5:64-75. doi: 10.1016/j.fob.2015.01.001. eCollection 2015. FEBS Open Bio. 2015. PMID: 25685665 Free PMC article.
-
The heart-specific NH2-terminal extension regulates the molecular conformation and function of cardiac troponin I.Am J Physiol Heart Circ Physiol. 2012 Feb 15;302(4):H923-33. doi: 10.1152/ajpheart.00637.2011. Epub 2011 Dec 2. Am J Physiol Heart Circ Physiol. 2012. PMID: 22140044 Free PMC article.
-
Protein Structure-Function Relationship at Work: Learning from Myopathy Mutations of the Slow Skeletal Muscle Isoform of Troponin T.Front Physiol. 2016 Oct 13;7:449. doi: 10.3389/fphys.2016.00449. eCollection 2016. Front Physiol. 2016. PMID: 27790152 Free PMC article. Review.
-
Effect of N-Terminal Extension of Cardiac Troponin I on the Ca(2+) Regulation of ATP Binding and ADP Dissociation of Myosin II in Native Cardiac Myofibrils.Biochemistry. 2016 Mar 29;55(12):1887-97. doi: 10.1021/acs.biochem.5b01059. Epub 2016 Mar 14. Biochemistry. 2016. PMID: 26862665 Free PMC article.
-
Gene regulation, alternative splicing, and posttranslational modification of troponin subunits in cardiac development and adaptation: a focused review.Front Physiol. 2014 Apr 30;5:165. doi: 10.3389/fphys.2014.00165. eCollection 2014. Front Physiol. 2014. PMID: 24817852 Free PMC article. Review.
References
-
- Gordon A. M., Homsher E., Regnier M. (2000) Physiol. Rev. 80, 853–924 - PubMed
-
- Perry S. V. (1998) J. Muscle Res. Cell Motil. 19, 575–602 - PubMed
-
- Perry S. V. (1999) Mol. Cell. Biochem. 190, 9–32 - PubMed
-
- Biesiadecki B. J., Jin J. P. (2002) J. Biol. Chem. 277, 18459–18468 - PubMed
-
- Biesiadecki B. J., Elder B. D., Yu Z. B., Jin J. P. (2002) J. Biol. Chem. 277, 50275–50285 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

