Actin cross-link assembly and disassembly mechanics for alpha-Actinin and fascin
- PMID: 20551315
- PMCID: PMC2924060
- DOI: 10.1074/jbc.M110.123117
Actin cross-link assembly and disassembly mechanics for alpha-Actinin and fascin
Abstract
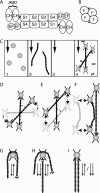
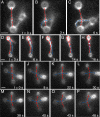
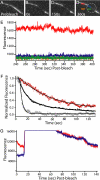
Self-assembly of complex structures is commonplace in biology but often poorly understood. In the case of the actin cytoskeleton, a great deal is known about the components that include higher order structures, such as lamellar meshes, filopodial bundles, and stress fibers. Each of these cytoskeletal structures contains actin filaments and cross-linking proteins, but the role of cross-linking proteins in the initial steps of structure formation has not been clearly elucidated. We employ an optical trapping assay to investigate the behaviors of two actin cross-linking proteins, fascin and alpha-actinin, during the first steps of structure assembly. Here, we show that these proteins have distinct binding characteristics that cause them to recognize and cross-link filaments that are arranged with specific geometries. alpha-Actinin is a promiscuous cross-linker, linking filaments over all angles. It retains this flexibility after cross-links are formed, maintaining a connection even when the link is rotated. Conversely, fascin is extremely selective, only cross-linking filaments in a parallel orientation. Surprisingly, bundles formed by either protein are extremely stable, persisting for over 0.5 h in a continuous wash. However, using fluorescence recovery after photobleaching and fluorescence decay experiments, we find that the stable fascin population can be rapidly competed away by free fascin. We present a simple avidity model for this cross-link dissociation behavior. Together, these results place constraints on how cytoskeletal structures assemble, organize, and disassemble in vivo.
Figures


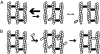
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

