Postendocytic sorting of constitutively internalized dopamine transporter in cell lines and dopaminergic neurons
- PMID: 20551317
- PMCID: PMC2930728
- DOI: 10.1074/jbc.M110.131003
Postendocytic sorting of constitutively internalized dopamine transporter in cell lines and dopaminergic neurons
Abstract
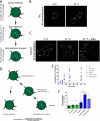
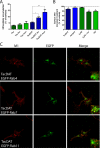
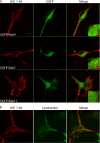
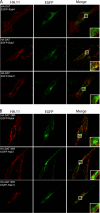
The dopamine transporter (DAT) mediates reuptake of released dopamine and is the target for psychostimulants, such as cocaine and amphetamine. DAT undergoes marked constitutive endocytosis, but little is known about the fate and sorting of the endocytosed transporter. To study DAT sorting in cells lines, we fused the one-transmembrane segment protein Tac to DAT, thereby generating a transporter (TacDAT) with an extracellular antibody epitope suited for trafficking studies. TacDAT was functional and endocytosed constitutively in HEK293 cells. According to an ELISA-based assay, TacDAT intracellular accumulation was increased by the lysosomal protease inhibitor leupeptin and by monensin, an inhibitor of lysosomal degradation and recycling. Monensin also reduced TacDAT surface expression consistent with partial recycling. In both HEK293 cells and in the dopaminergic cell line 1Rb3An27, constitutively internalized TacDAT displayed primary co-localization with the late endosomal marker Rab7, less co-localization with the "short loop" recycling marker Rab4, and little co-localization with the marker of "long loop" recycling endosomes, Rab11. Removal by mutation of N-terminal ubiquitination sites did not affect this sorting pattern. The sorting pattern was distinct from a bona fide recycling membrane protein, the beta(2)-adrenergic receptor, that co-localized primarily with Rab11 and Rab4. Constitutively internalized wild type DAT probed with the fluorescently tagged cocaine analogue JHC 1-64, exhibited the same co-localization pattern as TacDAT in 1Rb3An27 cells and in cultured midbrain dopaminergic neurons. We conclude that DAT is constitutively internalized and sorted in a ubiquitination-independent manner to late endosomes/lysosomes and in part to a Rab4 positive short loop recycling pathway.
Figures



Similar articles
-
Differential Internalization Rates and Postendocytic Sorting of the Norepinephrine and Dopamine Transporters Are Controlled by Structural Elements in the N Termini.J Biol Chem. 2016 Mar 11;291(11):5634-5651. doi: 10.1074/jbc.M115.702050. Epub 2016 Jan 19. J Biol Chem. 2016. PMID: 26786096 Free PMC article.
-
The serotonin transporter undergoes constitutive internalization and is primarily sorted to late endosomes and lysosomal degradation.J Biol Chem. 2014 Aug 15;289(33):23004-23019. doi: 10.1074/jbc.M113.495754. Epub 2014 Jun 27. J Biol Chem. 2014. PMID: 24973209 Free PMC article.
-
Differential targeting of the dopamine transporter to recycling or degradative pathways during amphetamine- or PKC-regulated endocytosis in dopamine neurons.FASEB J. 2013 Aug;27(8):2995-3007. doi: 10.1096/fj.12-218727. Epub 2013 Apr 23. FASEB J. 2013. PMID: 23612789 Free PMC article.
-
Regulation of G-protein-coupled receptor activity by rab GTPases.Recept Channels. 2002;8(2):87-97. Recept Channels. 2002. PMID: 12448790 Review.
-
Dopamine transporter endocytic trafficking: Neuronal mechanisms and potential impact on DA-dependent behaviors.J Neurochem. 2025 Jan;169(1):e16284. doi: 10.1111/jnc.16284. J Neurochem. 2025. PMID: 39655745 Review.
Cited by
-
Dynamic control of the dopamine transporter in neurotransmission and homeostasis.NPJ Parkinsons Dis. 2021 Mar 5;7(1):22. doi: 10.1038/s41531-021-00161-2. NPJ Parkinsons Dis. 2021. PMID: 33674612 Free PMC article. Review.
-
Activation of the cAMP/PKA pathway induces UT-A1 urea transporter monoubiquitination and targets it for lysosomal degradation.Am J Physiol Renal Physiol. 2013 Dec 15;305(12):F1775-82. doi: 10.1152/ajprenal.00393.2013. Epub 2013 Oct 16. Am J Physiol Renal Physiol. 2013. PMID: 24133116 Free PMC article.
-
Dopamine transporter trafficking and Rit2 GTPase: Mechanism of action and in vivo impact.J Biol Chem. 2020 Apr 17;295(16):5229-5244. doi: 10.1074/jbc.RA120.012628. Epub 2020 Mar 4. J Biol Chem. 2020. PMID: 32132171 Free PMC article.
-
Differential Internalization Rates and Postendocytic Sorting of the Norepinephrine and Dopamine Transporters Are Controlled by Structural Elements in the N Termini.J Biol Chem. 2016 Mar 11;291(11):5634-5651. doi: 10.1074/jbc.M115.702050. Epub 2016 Jan 19. J Biol Chem. 2016. PMID: 26786096 Free PMC article.
-
Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease.Pharmacol Rev. 2015 Oct;67(4):1005-24. doi: 10.1124/pr.114.010397. Pharmacol Rev. 2015. PMID: 26408528 Free PMC article. Review.
References
-
- Chen N. H., Reith M. E., Quick M. W. (2004) Pflugers Arch. 447, 519–531 - PubMed
-
- Gether U., Andersen P. H., Larsson O. M., Schousboe A. (2006) Trends Pharmacol. Sci. 27, 375–383 - PubMed
-
- Torres G. E., Amara S. G. (2007) Curr. Opin. Neurobiol. 17, 304–312 - PubMed
-
- Gainetdinov R. R., Caron M. G. (2003) Annu. Rev. Pharmacol. Toxicol. 43, 261–284 - PubMed
-
- Torres G. E., Gainetdinov R. R., Caron M. G. (2003) Nat. Rev. Neurosci. 4, 13–25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

