Sumoylation of bZIP transcription factor NRL modulates target gene expression during photoreceptor differentiation
- PMID: 20551322
- PMCID: PMC2919127
- DOI: 10.1074/jbc.M110.142810
Sumoylation of bZIP transcription factor NRL modulates target gene expression during photoreceptor differentiation
Abstract
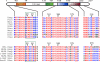
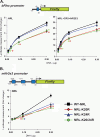
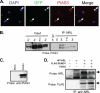
Development of rod photoreceptors in the mammalian retina is critically dependent on the basic motif-leucine zipper transcription factor NRL (neural retina leucine zipper). In the absence of NRL, photoreceptor precursors in mouse retina produce only cones that primarily express S-opsin. Conversely, ectopic expression of NRL in post-mitotic precursors leads to a rod-only retina. To explore the role of signaling molecules in modulating NRL function, we identified putative sites of post-translational modification in the NRL protein by in silico analysis. Here, we demonstrate the sumoylation of NRL in vivo and in vitro, with two small ubiquitin-like modifier (SUMO) molecules attached to the Lys-20 residue. NRL-K20R and NRL-K20R/K24R sumoylation mutants show reduced transcriptional activation of Nr2e3 and rhodopsin promoters (two direct targets of NRL) in reporter assays when compared with wild-type NRL. Consistent with this, in vivo electroporation of the NRL-K20R/K24R mutant into newborn Nrl(-/-) mouse retina leads to reduced Nr2e3 activation and only a partial rescue of the Nrl(-/-) phenotype in contrast to the wild-type NRL that is able to convert cones to rod photoreceptors. Although PIAS3 (protein inhibitor of activated STAT3), an E3-SUMO ligase implicated in photoreceptor differentiation, can be immunoprecipitated with NRL, there appears to be redundancy in E3 ligases, and PIAS3 does not seem to be essential for NRL sumoylation. Our studies suggest an important role of sumoylation in fine-tuning the activity of NRL and thereby incorporating yet another layer of control in gene regulatory networks involved in photoreceptor development and homeostasis.
Figures


Similar articles
-
Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors.Brain Res. 2008 Oct 21;1236:16-29. doi: 10.1016/j.brainres.2008.01.028. Epub 2008 Jan 18. Brain Res. 2008. PMID: 18294621 Free PMC article.
-
Transformation of cone precursors to functional rod photoreceptors by bZIP transcription factor NRL.Proc Natl Acad Sci U S A. 2007 Jan 30;104(5):1679-84. doi: 10.1073/pnas.0605934104. Epub 2007 Jan 22. Proc Natl Acad Sci U S A. 2007. PMID: 17242361 Free PMC article.
-
Combinatorial regulation of photoreceptor differentiation factor, neural retina leucine zipper gene NRL, revealed by in vivo promoter analysis.J Biol Chem. 2011 Aug 12;286(32):28247-55. doi: 10.1074/jbc.M111.257246. Epub 2011 Jun 14. J Biol Chem. 2011. PMID: 21673114 Free PMC article.
-
SUMO weighs in on a photoreceptor finish.Dev Cell. 2009 Feb;16(2):165-6. doi: 10.1016/j.devcel.2009.01.017. Dev Cell. 2009. PMID: 19217419 Review.
-
Regulation of photoreceptor gene expression by Crx-associated transcription factor network.Brain Res. 2008 Feb 4;1192:114-33. doi: 10.1016/j.brainres.2007.06.036. Epub 2007 Jun 30. Brain Res. 2008. PMID: 17662965 Free PMC article. Review.
Cited by
-
Expression of deubiquitinating enzyme genes in the developing mammal retina.Mol Vis. 2019 Dec 2;25:800-813. eCollection 2019. Mol Vis. 2019. PMID: 31819342 Free PMC article.
-
SUMO1-regulated DBC1 promotes p53-dependent stress-induced apoptosis of lens epithelial cells.Aging (Albany NY). 2023 Sep 7;15(17):8812-8832. doi: 10.18632/aging.205001. Epub 2023 Sep 7. Aging (Albany NY). 2023. PMID: 37683133 Free PMC article.
-
Posttranslational Modification of Sox11 Regulates RGC Survival and Axon Regeneration.eNeuro. 2021 Feb 11;8(1):ENEURO.0358-20.2020. doi: 10.1523/ENEURO.0358-20.2020. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33441400 Free PMC article.
-
Emerging roles of the SUMO pathway in development.Cell Mol Life Sci. 2011 Dec;68(24):4045-64. doi: 10.1007/s00018-011-0792-5. Epub 2011 Sep 4. Cell Mol Life Sci. 2011. PMID: 21892772 Free PMC article. Review.
-
Reprogramming amacrine and photoreceptor progenitors into retinal ganglion cells by replacing Neurod1 with Atoh7.Development. 2013 Feb 1;140(3):541-51. doi: 10.1242/dev.085886. Development. 2013. PMID: 23293286 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

