Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4)
- PMID: 20551324
- PMCID: PMC2924030
- DOI: 10.1074/jbc.M110.103127
Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4)
Abstract
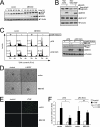
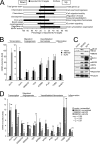
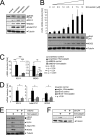
The MEK5/Erk5 MAPK cascade has recently been implicated in the regulation of endothelial integrity and represents a candidate pathway mediating the beneficial effects of laminar flow, a major factor preventing vascular dysfunction and disease. Here we expressed a constitutively active mutant of MEK5 (MEK5D) to study the transcriptional and functional responses to Erk5 activation in human primary endothelial cells. We provide evidence that constitutive Erk5 activation elicits an overall protective phenotype characterized by increased apoptosis resistance and a decreased angiogenic, migratory, and inflammatory potential. This is supported by bioinformatic microarray analysis, which uncovered a statistical overrepresentation of corresponding functional clusters as well as a significant induction of anti-thrombotic, hemostatic, and vasodilatory genes. We identify KLF4 as a novel Erk5 target and demonstrate a critical role of this transcription factor downstream of Erk5. We show that KLF4 expression largely reproduces the protective phenotype in endothelial cells, whereas KLF4 siRNA suppresses expression of various Erk5 targets. Additionally, we show that vasoprotective statins potently induce KLF4 and KLF4-dependent gene expression via activation of Erk5. Our data underscore a major protective function of the MEK5/Erk5/KLF4 module in ECs and implicate agonistic Erk5 activation as potential strategy for treatment of vascular diseases.
Figures



Similar articles
-
Erk5 inhibits endothelial migration via KLF2-dependent down-regulation of PAK1.Cardiovasc Res. 2015 Jan 1;105(1):86-95. doi: 10.1093/cvr/cvu236. Epub 2014 Nov 10. Cardiovasc Res. 2015. PMID: 25388666
-
MEK5 is activated by shear stress, activates ERK5 and induces KLF4 to modulate TNF responses in human dermal microvascular endothelial cells.Microcirculation. 2011 Feb;18(2):102-17. doi: 10.1111/j.1549-8719.2010.00071.x. Microcirculation. 2011. PMID: 21166929 Free PMC article.
-
Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells.Biochem Biophys Res Commun. 2010 Jan 1;391(1):984-9. doi: 10.1016/j.bbrc.2009.12.002. Epub 2009 Dec 5. Biochem Biophys Res Commun. 2010. PMID: 19968965 Free PMC article.
-
The MEK5/ERK5 Pathway in Health and Disease.Int J Mol Sci. 2021 Jul 15;22(14):7594. doi: 10.3390/ijms22147594. Int J Mol Sci. 2021. PMID: 34299213 Free PMC article. Review.
-
The MEK5/ERK5 signalling pathway in cancer: a promising novel therapeutic target.Drug Discov Today. 2016 Oct;21(10):1654-1663. doi: 10.1016/j.drudis.2016.06.010. Epub 2016 Jun 16. Drug Discov Today. 2016. PMID: 27320690 Review.
Cited by
-
MicroRNA-7 Deficiency Ameliorates the Pathologies of Acute Lung Injury through Elevating KLF4.Front Immunol. 2016 Oct 7;7:389. doi: 10.3389/fimmu.2016.00389. eCollection 2016. Front Immunol. 2016. PMID: 27774091 Free PMC article.
-
The Role of Protein SUMOylation in the Pathogenesis of Atherosclerosis.J Clin Med. 2019 Nov 2;8(11):1856. doi: 10.3390/jcm8111856. J Clin Med. 2019. PMID: 31684100 Free PMC article. Review.
-
The decrease in histone methyltransferase EZH2 in response to fluid shear stress alters endothelial gene expression and promotes quiescence.Angiogenesis. 2016 Jan;19(1):9-24. doi: 10.1007/s10456-015-9485-2. Epub 2015 Sep 28. Angiogenesis. 2016. PMID: 26416763 Free PMC article.
-
MicroRNA 139-5p coordinates APLNR-CXCR4 crosstalk during vascular maturation.Nat Commun. 2016 Apr 12;7:11268. doi: 10.1038/ncomms11268. Nat Commun. 2016. PMID: 27068353 Free PMC article.
-
MEF2 (Myocyte Enhancer Factor 2) Is Essential for Endothelial Homeostasis and the Atheroprotective Gene Expression Program.Arterioscler Thromb Vasc Biol. 2021 Mar;41(3):1105-1123. doi: 10.1161/ATVBAHA.120.314978. Epub 2021 Jan 7. Arterioscler Thromb Vasc Biol. 2021. PMID: 33406884 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

