Proteolysis-induced N-terminal ectodomain shedding of the integral membrane glycoprotein CUB domain-containing protein 1 (CDCP1) is accompanied by tyrosine phosphorylation of its C-terminal domain and recruitment of Src and PKCdelta
- PMID: 20551327
- PMCID: PMC2924022
- DOI: 10.1074/jbc.M109.096453
Proteolysis-induced N-terminal ectodomain shedding of the integral membrane glycoprotein CUB domain-containing protein 1 (CDCP1) is accompanied by tyrosine phosphorylation of its C-terminal domain and recruitment of Src and PKCdelta
Abstract
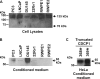
CUB-domain-containing protein 1 (CDCP1) is an integral membrane glycoprotein with potential as a marker and therapeutic target for a number of cancers. Here we examine mechanisms regulating cellular processing of CDCP1. By analyzing cell lines exclusively passaged non-enzymatically and through use of a panel of protease inhibitors, we demonstrate that full-length 135 kDa CDCP1 is post-translationally processed in a range of cell lines by a mechanism involving serine protease activity, generating a C-terminal 70-kDa fragment. Immunopurification and N-terminal sequencing of this cell-retained fragment and detailed mutagenesis, show that proteolytic processing of CDCP1 occurs at two sites, Arg-368 and Lys-369. We show that the serine protease matriptase is an efficient, but not essential, cellular processor of CDCP1 at Arg-368. Importantly, we also demonstrate that proteolysis induces tyrosine phosphorylation of 70-kDa CDCP1 and recruitment of Src and PKCdelta to this fragment. In addition, Western blot and mass spectroscopy analyses show that an N-terminal 65-kDa CDCP1 ectodomain is shed intact from the cell surface. These data provide new insights into mechanisms regulating CDCP1 and suggest that the biological role of this protein and, potentially, its function in cancer, may be mediated by both 70-kDa cell retained and 65-kDa shed fragments, as well as the full-length 135-kDa protein.
Figures







Similar articles
-
CUB domain-containing protein 1, a prognostic factor for human pancreatic cancers, promotes cell migration and extracellular matrix degradation.Cancer Res. 2010 Jun 15;70(12):5136-46. doi: 10.1158/0008-5472.CAN-10-0220. Epub 2010 May 25. Cancer Res. 2010. PMID: 20501830
-
Blocking of CDCP1 cleavage in vivo prevents Akt-dependent survival and inhibits metastatic colonization through PARP1-mediated apoptosis of cancer cells.Oncogene. 2012 Aug 30;31(35):3924-38. doi: 10.1038/onc.2011.555. Epub 2011 Dec 19. Oncogene. 2012. PMID: 22179830 Free PMC article.
-
Suppression of autophagy by CUB domain-containing protein 1 signaling is essential for anchorage-independent survival of lung cancer cells.Cancer Sci. 2013 Jul;104(7):865-70. doi: 10.1111/cas.12154. Epub 2013 Apr 19. Cancer Sci. 2013. PMID: 23510015 Free PMC article.
-
The cell surface glycoprotein CDCP1 in cancer--insights, opportunities, and challenges.IUBMB Life. 2009 Jul;61(7):723-30. doi: 10.1002/iub.198. IUBMB Life. 2009. PMID: 19514048 Review.
-
Roles of CUB domain-containing protein 1 signaling in cancer invasion and metastasis.Cancer Sci. 2011 Nov;102(11):1943-8. doi: 10.1111/j.1349-7006.2011.02052.x. Epub 2011 Sep 6. Cancer Sci. 2011. PMID: 21812858 Review.
Cited by
-
Evaluation of antibodies directed against human protease-activated receptor-2.Naunyn Schmiedebergs Arch Pharmacol. 2012 Sep;385(9):861-73. doi: 10.1007/s00210-012-0783-6. Epub 2012 Jul 31. Naunyn Schmiedebergs Arch Pharmacol. 2012. PMID: 22842724
-
The cell surface glycoprotein CUB domain-containing protein 1 (CDCP1) contributes to epidermal growth factor receptor-mediated cell migration.J Biol Chem. 2012 Mar 23;287(13):9792-9803. doi: 10.1074/jbc.M111.335448. Epub 2012 Feb 7. J Biol Chem. 2012. PMID: 22315226 Free PMC article.
-
CUB Domain-Containing Protein 1 (CDCP1) Is a Target for Radioligand Therapy in Castration-Resistant Prostate Cancer, including PSMA Null Disease.Clin Cancer Res. 2022 Jul 15;28(14):3066-3075. doi: 10.1158/1078-0432.CCR-21-3858. Clin Cancer Res. 2022. PMID: 35604681 Free PMC article.
-
Identification of CDCP1 as a hypoxia-inducible factor 2α (HIF-2α) target gene that is associated with survival in clear cell renal cell carcinoma patients.Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3483-8. doi: 10.1073/pnas.1222435110. Epub 2013 Feb 1. Proc Natl Acad Sci U S A. 2013. PMID: 23378636 Free PMC article.
-
Repurposing Tranexamic Acid as an Anticancer Agent.Front Pharmacol. 2022 Jan 13;12:792600. doi: 10.3389/fphar.2021.792600. eCollection 2021. Front Pharmacol. 2022. PMID: 35095503 Free PMC article.
References
-
- Scherl-Mostageer M., Sommergruber W., Abseher R., Hauptmann R., Ambros P., Schweifer N. (2001) Oncogene 20, 4402–4408 - PubMed
-
- Hooper J. D., Zijlstra A., Aimes R. T., Liang H., Claassen G. F., Tarin D., Testa J. E., Quigley J. P. (2003) Oncogene 22, 1783–1794 - PubMed
-
- Brown T. A., Yang T. M., Zaitsevskaia T., Xia Y., Dunn C. A., Sigle R. O., Knudsen B., Carter W. G. (2004) J. Biol. Chem. 279, 14772–14783 - PubMed
-
- Bühring H. J., Kuçi S., Conze T., Rathke G., Bartolović K., Grünebach F., Scherl-Mostageer M., Brümmendorf T. H., Schweifer N., Lammers R. (2004) Stem. Cells 22, 334–343 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

