Critical roles of hydrophobicity and orientation of side chains for inactivation of sarcoplasmic reticulum Ca2+-ATPase with thapsigargin and thapsigargin analogs
- PMID: 20551329
- PMCID: PMC2937915
- DOI: 10.1074/jbc.M110.136242
Critical roles of hydrophobicity and orientation of side chains for inactivation of sarcoplasmic reticulum Ca2+-ATPase with thapsigargin and thapsigargin analogs
Abstract
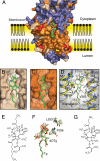
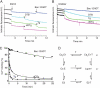
Thapsigargin (Tg), a specific inhibitor of sarco/endoplasmic Ca(2+)-ATPases (SERCA), binds with high affinity to the E2 conformation of these ATPases. SERCA inhibition leads to elevated calcium levels in the cytoplasm, which in turn induces apoptosis. We present x-ray crystallographic and intrinsic fluorescence data to show how Tg and chemical analogs of the compound with modified or removed side chains bind to isolated SERCA 1a membranes. This occurs by uptake via the membrane lipid followed by insertion into a resident intramembranous binding site with few adaptative changes. Our binding data indicate that a balanced hydrophobicity and accurate positioning of the side chains, provided by the central guaianolide ring structure, defines a pharmacophore of Tg that governs both high affinity and access to the protein-binding site. Tg analogs substituted with long linkers at O-8 extend from the binding site between transmembrane segments to the putative N-terminal Ca(2+) entry pathway. The long chain analogs provide a rational basis for the localization of the linker, the presence of which is necessary for enabling prostate-specific antigen to cleave peptide-conjugated prodrugs targeting SERCA of cancer cells (Denmeade, S. R., Jakobsen, C. M., Janssen, S., Khan, S. R., Garrett, E. S., Lilja, H., Christensen, S. B., and Isaacs, J. T. (2003) J. Natl. Cancer Inst. 95, 990-1000). Our study demonstrates the usefulness of a simple in vitro system to test and direct development toward the formulation of new Tg derivatives with improved properties for SERCA targeting. Finally, we propose that the Tg binding pocket may be a regulatory site that, for example, is sensitive to cholesterol.
Figures



Similar articles
-
Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response.J Biol Chem. 2017 Dec 1;292(48):19656-19673. doi: 10.1074/jbc.M117.796920. Epub 2017 Sep 29. J Biol Chem. 2017. PMID: 28972171 Free PMC article.
-
Design and total synthesis of unnatural analogues of the sub-nanomolar SERCA inhibitor thapsigargin.Org Biomol Chem. 2007 May 7;5(9):1427-36. doi: 10.1039/b702481a. Epub 2007 Mar 23. Org Biomol Chem. 2007. PMID: 17464412
-
Molecular determinants of thapsigargin binding by SERCA Ca2+-ATPase: a computational docking study.Proteins. 2004 Aug 15;56(3):595-606. doi: 10.1002/prot.20105. Proteins. 2004. PMID: 15229891
-
Linking Biochemical and Structural States of SERCA: Achievements, Challenges, and New Opportunities.Int J Mol Sci. 2020 Jun 10;21(11):4146. doi: 10.3390/ijms21114146. Int J Mol Sci. 2020. PMID: 32532023 Free PMC article. Review.
-
Targeting thapsigargin towards tumors.Steroids. 2015 May;97:2-7. doi: 10.1016/j.steroids.2014.07.009. Epub 2014 Jul 24. Steroids. 2015. PMID: 25065587 Free PMC article. Review.
Cited by
-
Tracing cytoplasmic Ca(2+) ion and water access points in the Ca(2+)-ATPase.Biophys J. 2012 Jan 18;102(2):268-77. doi: 10.1016/j.bpj.2011.12.009. Biophys J. 2012. PMID: 22339863 Free PMC article.
-
Chemical composition, antibacterial, antioxidant and insecticidal activities of moroccan Thapsia transtagana essential oil.Saudi J Biol Sci. 2021 Dec;28(12):6756-6764. doi: 10.1016/j.sjbs.2021.07.052. Epub 2021 Jul 24. Saudi J Biol Sci. 2021. PMID: 34866974 Free PMC article.
-
Cardiolipin interaction with subunit c of ATP synthase: solid-state NMR characterization.Biochim Biophys Acta. 2015 Jan;1848(1 Pt B):260-5. doi: 10.1016/j.bbamem.2014.08.021. Epub 2014 Aug 25. Biochim Biophys Acta. 2015. PMID: 25168468 Free PMC article.
-
Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response.J Biol Chem. 2017 Dec 1;292(48):19656-19673. doi: 10.1074/jbc.M117.796920. Epub 2017 Sep 29. J Biol Chem. 2017. PMID: 28972171 Free PMC article.
-
Targeting oncogenic Notch signaling with SERCA inhibitors.J Hematol Oncol. 2021 Jan 6;14(1):8. doi: 10.1186/s13045-020-01015-9. J Hematol Oncol. 2021. PMID: 33407740 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

