Morphological characterization and fusion properties of triglyceride-rich lipoproteins obtained from cells transduced with hepatitis C virus glycoproteins
- PMID: 20551330
- PMCID: PMC2919142
- DOI: 10.1074/jbc.M110.131664
Morphological characterization and fusion properties of triglyceride-rich lipoproteins obtained from cells transduced with hepatitis C virus glycoproteins
Abstract
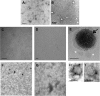
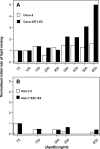
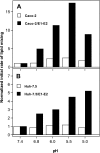
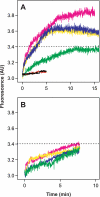
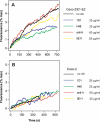
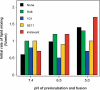
The density of hepatitis C virus (HCV) particles circulating in the blood of chronically infected patients and of cell-culture produced HCV is heterogeneous. Specific infectivity and fusion of low density particles are higher than those of high density particles. We recently characterized hybrid particles produced by Caco-2 colon or Huh-7.5 liver cells transduced with HCV E1 and E2 envelope glycoproteins. Caco-2-derived particles, called empty lipo-viral particles (eLVP), are composed of triglyceride-rich lipoproteins positive for apolipoproteins B (i.e. apoB100 and apoB48) and contain HCV E1 and E2. Here we aimed at characterizing the morphology and in vitro fusion properties of eLVP using electron microscopy and fluorescence spectroscopy. They displayed the aspect of beta-lipoproteins, and immunogold labeling confirmed the presence of apoB and HCV E1 and E2 at their surface. These particles are able to fuse with lipid bilayers (liposomes) in a fusion process leading to the coalescence of internal contents of triglyceride-rich lipoproteins particles and liposomes. Fusion was pH-dependent and could be inhibited by either Z-fFG, a peptide known to inhibit viral fusion, or by monoclonal antibodies directed against HCV E2 or the apolipoprotein moiety of the hybrid particle. Interestingly, particles derived from Huh-7.5 cells failed to display equivalent efficient fusion. Optimal fusion activity is, thus, observed when HCV envelope proteins are associated to apoB-positive hybrid particles. Our results, therefore, point to a crucial role of the E1 and E2 proteins in HCV fusion with a subtle interplay with the apolipoprotein part of eLVP.
Figures

Similar articles
-
Secretion of hepatitis C virus envelope glycoproteins depends on assembly of apolipoprotein B positive lipoproteins.PLoS One. 2009;4(1):e4233. doi: 10.1371/journal.pone.0004233. Epub 2009 Jan 21. PLoS One. 2009. PMID: 19156195 Free PMC article.
-
A serum protein factor mediates maturation and apoB-association of HCV particles in the extracellular milieu.J Hepatol. 2019 Apr;70(4):626-638. doi: 10.1016/j.jhep.2018.11.033. Epub 2018 Dec 14. J Hepatol. 2019. PMID: 30553840
-
Morphological identification of hepatitis C virus E1 and E2 envelope glycoproteins on the virion surface using immunogold electron microscopy.Int J Mol Med. 2006 Oct;18(4):673-8. Int J Mol Med. 2006. PMID: 16964422
-
Lipids: a key for hepatitis C virus entry and a potential target for antiviral strategies.Biochimie. 2013 Jan;95(1):96-102. doi: 10.1016/j.biochi.2012.07.016. Epub 2012 Aug 1. Biochimie. 2013. PMID: 22884392 Review.
-
Incorporation of hepatitis C virus E1 and E2 glycoproteins: the keystones on a peculiar virion.Viruses. 2014 Mar 11;6(3):1149-87. doi: 10.3390/v6031149. Viruses. 2014. PMID: 24618856 Free PMC article. Review.
Cited by
-
Hepatitis C virus, cholesterol and lipoproteins--impact for the viral life cycle and pathogenesis of liver disease.Viruses. 2013 May 23;5(5):1292-324. doi: 10.3390/v5051292. Viruses. 2013. PMID: 23698400 Free PMC article. Review.
-
Lipoprotein receptors and lipid enzymes in hepatitis C virus entry and early steps of infection.Scientifica (Cairo). 2012;2012:709853. doi: 10.6064/2012/709853. Epub 2012 Dec 23. Scientifica (Cairo). 2012. PMID: 24278733 Free PMC article. Review.
-
Characterization of the envelope glycoproteins associated with infectious hepatitis C virus.J Virol. 2010 Oct;84(19):10159-68. doi: 10.1128/JVI.01180-10. Epub 2010 Jul 28. J Virol. 2010. PMID: 20668082 Free PMC article.
-
Entry inhibitors: New advances in HCV treatment.Emerg Microbes Infect. 2016 Jan 6;5(1):e3. doi: 10.1038/emi.2016.3. Emerg Microbes Infect. 2016. PMID: 26733381 Free PMC article. Review.
-
Hepatitis C virus entry into the hepatocyte.Cent Eur J Biol. 2011;6(6):933-945. doi: 10.2478/s11535-011-0076-y. Epub 2011 Nov 7. Cent Eur J Biol. 2011. PMID: 32215118 Free PMC article. Review.
References
-
- Lindenbach B. D., Rice C. M. (2001) in Fields Virology (Knipe D. M., Howley P. M. eds) Vol. 1, pp. 991–1041, Lippincott-Raven, Philadelphia
-
- Moradpour D., Penin F., Rice C. M. (2007) Nat. Rev. Microbiol. 5, 453–463 - PubMed
-
- Gottwein J. M., Scheel T. K., Jensen T. B., Lademann J. B., Prentoe J. C., Knudsen M. L., Hoegh A. M., Bukh J. (2009) Hepatology 49, 364–377 - PubMed
-
- Murphy D., Chamberland J., Dandavino R., Sablon E. (2007) Hepatology 46, Suppl. 1, 623
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

