Efficient and stable MGMT-mediated selection of long-term repopulating stem cells in nonhuman primates
- PMID: 20551514
- PMCID: PMC2898586
- DOI: 10.1172/JCI40767
Efficient and stable MGMT-mediated selection of long-term repopulating stem cells in nonhuman primates
Abstract
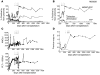
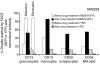
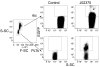
HSC transplantation using genetically modified autologous cells is a promising therapeutic strategy for various genetic diseases, cancer, and HIV. However, for many of these conditions, the current efficiency of gene transfer to HSCs is not sufficient for clinical use. The ability to increase the percentage of gene-modified cells following transplantation is critical to overcoming this obstacle. In vivo selection with mutant methylguanine methyltransferase (MGMTP140K) has been proposed to overcome low gene transfer efficiency to HSCs. Previous studies have shown efficient in vivo selection in mice and dogs but only transient selection in primates. Here, we report efficient and stable MGMTP140K-mediated multilineage selection in both macaque and baboon nonhuman primate models. Treatment consisting of both O6-benzylguanine (O6BG) and N,N'-bis(2-chloroethyl)-N-nitroso-urea (BCNU) stably increased the percentage of transgene-expressing cells from a range of initial levels of engrafted genetically modified cells, with the longest follow-up after drug treatment occurring over 2.2 years. Drug treatment was well tolerated, and selection occurred in myeloid, lymphoid, and erythroid cells as well as platelets. Retrovirus integration site analysis before and after drug treatments confirmed the presence of multiple clones. These nonhuman primate studies closely model a clinical setting and should have broad applications for HSC gene therapy targeting human diseases of malignant, genetic, and infectious nature, including HIV.
Figures


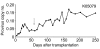
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

