Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury
- PMID: 20551909
- PMCID: PMC2956932
- DOI: 10.1038/mt.2010.100
Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury
Abstract
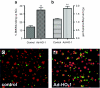
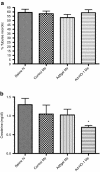
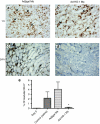
Acute kidney injury has a high mortality and lacks specific therapies, with ischemia/reperfusion injury (IRI) being the predominant cause. Macrophages (M phi) have been used successfully in cell therapy to deliver targeted therapeutic genes in models of inflammatory kidney disease. Heme oxygenase-1 (HO-1) catalyzes heme breakdown and has important cytoprotective functions. We hypothesized that administration of M phi modified to overexpress HO-1 would protect from renal IRI. Using an adenoviral construct (Ad-HO-1), HO-1 was overexpressed in primary bone marrow-derived M phi (BMDM). In vitro Ad-HO-1 M phi showed an anti-inflammatory phenotype with increased phagocytosis of apoptotic cells (ACs) and increased interleukin (IL)-10 but reduced TNF-alpha and nitric oxide (NO) following lipopolysaccharide/interferon-gamma (IFN gamma) stimulation compared to control transduced or unmodified M phi. In vivo, intravenously (IV) injected M phi homed preferentially to the post-IRI kidney compared to uninjured control following experimental IRI. At 24 hours postinjury, despite equivalent levels of tubular necrosis, apoptosis, and capillary density between groups, the injection of Ad-HO-1 M phi resulted in preserved renal function (serum creatinine reduced by 46%), and reduced microvascular platelet deposition. These data demonstrate that genetically modified M phi improve the outcomes in IRI when administered after the establishment of structural injury, raising the prospect of targeted cell therapy to support the function of the acutely injured kidney.
Figures

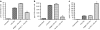


References
-
- Thadhani R, Pascual M., and, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. - PubMed
-
- Bonventre JV., and, Zuk A. Ischemic acute renal failure: an inflammatory disease. Kidney Int. 2004;66:480–485. - PubMed
-
- Day YJ, Huang L, Ye H, Linden J., and, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol. 2005;288:F722–F731. - PubMed
-
- Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W., and, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol. 2003;171:3210–3215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

