CaMKII hunkers down on the muscarinic M4 receptor to help curb cocaine-induced hyperlocomotion
- PMID: 20551968
- PMCID: PMC2892364
- DOI: 10.1038/emboj.2010.105
CaMKII hunkers down on the muscarinic M4 receptor to help curb cocaine-induced hyperlocomotion
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
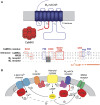
Comment on
-
CaMKIIalpha interacts with M4 muscarinic receptors to control receptor and psychomotor function.EMBO J. 2010 Jun 16;29(12):2070-81. doi: 10.1038/emboj.2010.93. Epub 2010 May 11. EMBO J. 2010. PMID: 20461055 Free PMC article.
References
-
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411: 801–805 - PubMed
-
- Jeon J, Dencker D, Wortwein G, Woldbye DP, Cui Y, Davis AA, Levey AI, Schutz G, Sager TN, Mork A, Li C, Deng CX, Fink-Jensen A, Wess J (2010) A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci 30: 2396–2405 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

