A retinoic acid receptor agonist Am80 rescues neurons, attenuates inflammatory reactions, and improves behavioral recovery after intracerebral hemorrhage in mice
- PMID: 20551971
- PMCID: PMC3049486
- DOI: 10.1038/jcbfm.2010.80
A retinoic acid receptor agonist Am80 rescues neurons, attenuates inflammatory reactions, and improves behavioral recovery after intracerebral hemorrhage in mice
Abstract
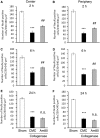
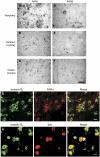
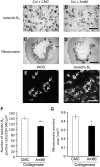
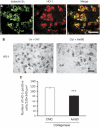
Am80 (tamibarotene) is a retinoic acid receptor (RAR) agonist clinically available for treatment of acute promyelocytic leukemia. As intracerebral hemorrhage (ICH) accompanies inflammatory reactions in the brain and also because retinoids may suppress activation of microglia, we investigated the effect of Am80 on collagenase-induced experimental model of ICH in adult mice. Daily oral administration of Am80 (5 mg/kg) starting from 1 day before or from up to 6 hours after intrastriatal injection of collagenase significantly inhibited the decrease in the number of striatal neurons at 3 days after the insult. Am80 showed no significant effect on the hematoma size and the extent of edema associated with hemorrhage. Prominent expression of RARα was observed in activated microglia/macrophages, and the number of activated microglia/macrophages in the perihematoma region was lower in Am80-treated mice than in vehicle-treated mice. Am80 treatment also reduced areas affected by hemorrhage-associated oxidative stress as indicated by nitrotyrosine immunoreactivity, and attenuated heme oxygenase-1 expression in activated microglia/macrophages. Moreover, Am80-treated mice exhibited better recovery from hemorrhage-induced neurologic deficits than vehicle-treated mice. These results suggest that RAR is a promising target of neuroprotective therapy for ICH.
Figures



Similar articles
-
Natural and synthetic retinoids afford therapeutic effects on intracerebral hemorrhage in mice.Eur J Pharmacol. 2012 May 15;683(1-3):125-31. doi: 10.1016/j.ejphar.2012.03.023. Epub 2012 Mar 23. Eur J Pharmacol. 2012. PMID: 22465180
-
Suppression of CXCL2 upregulation underlies the therapeutic effect of the retinoid Am80 on intracerebral hemorrhage in mice.J Neurosci Res. 2014 Aug;92(8):1024-34. doi: 10.1002/jnr.23379. Epub 2014 Mar 21. J Neurosci Res. 2014. PMID: 24659080
-
Retinoic acid receptor stimulation protects midbrain dopaminergic neurons from inflammatory degeneration via BDNF-mediated signaling.J Neurochem. 2009 Jul;110(2):707-18. doi: 10.1111/j.1471-4159.2009.06171.x. Epub 2009 May 15. J Neurochem. 2009. PMID: 19457078
-
Tamibarotene: a candidate retinoid drug for Alzheimer's disease.Biol Pharm Bull. 2012;35(8):1206-12. doi: 10.1248/bpb.b12-00314. Biol Pharm Bull. 2012. PMID: 22863914 Review.
-
Hematology and inflammatory signaling of intracerebral hemorrhage.Stroke. 2013 Jun;44(6 Suppl 1):S74-8. doi: 10.1161/STROKEAHA.111.000662. Stroke. 2013. PMID: 23709738 Free PMC article. Review. No abstract available.
Cited by
-
Decreased Serum Retinoic Acid May Predict Poor Outcome in Ischemic Stroke Patients.Neuropsychiatr Dis Treat. 2020 Jun 10;16:1483-1491. doi: 10.2147/NDT.S254591. eCollection 2020. Neuropsychiatr Dis Treat. 2020. PMID: 32606701 Free PMC article.
-
Nuclear receptors of NR1 and NR4 subfamilies in the regulation of microglial functions and pathology.Pharmacol Res Perspect. 2021 Dec;9(6):e00766. doi: 10.1002/prp2.766. Pharmacol Res Perspect. 2021. PMID: 34676987 Free PMC article. Review.
-
Menaquinone-4 Alleviates Neurological Deficits Associated with Intracerebral Hemorrhage by Preserving Corticospinal Tract in Mice.Neurochem Res. 2024 Jul;49(7):1838-1850. doi: 10.1007/s11064-024-04150-8. Epub 2024 May 10. Neurochem Res. 2024. PMID: 38727984
-
Ergosta-7,9(11),22-trien-3β-ol Alleviates Intracerebral Hemorrhage-Induced Brain Injury and BV-2 Microglial Activation.Molecules. 2021 May 17;26(10):2970. doi: 10.3390/molecules26102970. Molecules. 2021. PMID: 34067678 Free PMC article.
-
Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway.Sci Rep. 2017 Apr 6;7(1):717. doi: 10.1038/s41598-017-00851-z. Sci Rep. 2017. PMID: 28386082 Free PMC article.
References
-
- Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27:325–355. - PubMed
-
- Chu K, Kim M, Jung KH, Jeon D, Lee ST, Kim J, Jeong SW, Kim SU, Lee SK, Shin HS, Roh JK. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004;1023:213–221. - PubMed
-
- Dheen ST, Jun Y, Yan Z, Tay SS, Ling EA. Retinoic acid inhibits expression of TNF-α and iNOS in activated rat microglia. Glia. 2005;50:21–31. - PubMed
-
- James ML, Warner DS, Laskowitz DT. Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care. 2008;9:139–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

