Radiotherapy suppresses angiogenesis in mice through TGF-betaRI/ALK5-dependent inhibition of endothelial cell sprouting
- PMID: 20552031
- PMCID: PMC2884035
- DOI: 10.1371/journal.pone.0011084
Radiotherapy suppresses angiogenesis in mice through TGF-betaRI/ALK5-dependent inhibition of endothelial cell sprouting
Abstract
Background: Radiotherapy is widely used to treat cancer. While rapidly dividing cancer cells are naturally considered the main target of radiotherapy, emerging evidence indicates that radiotherapy also affects endothelial cell functions, and possibly also their angiogenic capacity. In spite of its clinical relevance, such putative anti-angiogenic effect of radiotherapy has not been thoroughly characterized. We have investigated the effect of ionizing radiation on angiogenesis using in vivo, ex vivo and in vitro experimental models in combination with genetic and pharmacological interventions.
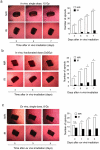
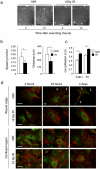
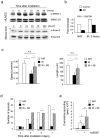
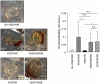
Principal findings: Here we show that high doses ionizing radiation locally suppressed VEGF- and FGF-2-induced Matrigel plug angiogenesis in mice in vivo and prevented endothelial cell sprouting from mouse aortic rings following in vivo or ex vivo irradiation. Quiescent human endothelial cells exposed to ionizing radiation in vitro resisted apoptosis, demonstrated reduced sprouting, migration and proliferation capacities, showed enhanced adhesion to matrix proteins, and underwent premature senescence. Irradiation induced the expression of P53 and P21 proteins in endothelial cells, but p53 or p21 deficiency and P21 silencing did not prevent radiation-induced inhibition of sprouting or proliferation. Radiation induced Smad-2 phosphorylation in skin in vivo and in endothelial cells in vitro. Inhibition of the TGF-beta type I receptor ALK5 rescued deficient endothelial cell sprouting and migration but not proliferation in vitro and restored defective Matrigel plug angiogenesis in irradiated mice in vivo. ALK5 inhibition, however, did not rescue deficient proliferation. Notch signaling, known to hinder angiogenesis, was activated by radiation but its inhibition, alone or in combination with ALK5 inhibition, did not rescue suppressed proliferation.
Conclusions: These results demonstrate that irradiation of quiescent endothelial cells suppresses subsequent angiogenesis and that ALK5 is a critical mediator of this suppression. These results extend our understanding of radiotherapy-induced endothelial dysfunctions, relevant to both therapeutic and unwanted effects of radiotherapy.
Conflict of interest statement
Figures





References
-
- Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4:737–747. - PubMed
-
- Rosen EM, Fan S, Rockwell S, Goldberg ID. The molecular and cellular basis of radiosensitivity: implications for understanding how normal tissues and tumors respond to therapeutic radiation. Cancer Invest. 1999;17:56–72. - PubMed
-
- Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. - PubMed
-
- Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. - PubMed
-
- Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005;44:13–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

