The effect on intracytoplasmic sperm injection outcome of genotype, male germ cell stage and freeze-thawing in mice
- PMID: 20552034
- PMCID: PMC2884038
- DOI: 10.1371/journal.pone.0011062
The effect on intracytoplasmic sperm injection outcome of genotype, male germ cell stage and freeze-thawing in mice
Abstract
Background: Intracytoplasmic sperm injection (ICSI) has been widely used to study the mechanisms of mammalian fertilization and to rescue male-factor infertility in humans and animals. However, very few systematic analyses have been conducted to define factors affecting the efficiency of ICSI. In this study, we undertook a large-scale series of ICSI experiments in mice to define the factors that might affect outcomes.
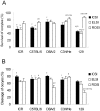
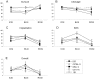
Methodology/principal findings: We used a 5 x 3 x 2 factorial design with the following factors: mouse genotype (ICR, C57BL/6, DBA/2, C3H/He, and 129/Sv strains), type of male germ cells (epididymal sperm, elongated or round spermatids), and their freeze-thawing treatment. The efficiencies (parameters) of each developmental step were analyzed by three-way ANOVA (significance level P<0.01). The type of male germ cells affected all the four parameters observed: oocyte survival after injection, cleavage of oocytes, implantation, and birth of offspring. Genotype affected the oocyte survival, cleavage and birth rates, whereas freeze-thawing had no effects on any of the parameters. There were significant genotype/cell type interactions for oocyte survival and cleavage, indicating that they were determined by a combination of strain and germ cell maturity. Multiple comparisons revealed that spermatozoa and elongated spermatids gave better implantation and birth rates than did round spermatids, while spermatozoa and elongated spermatozoa were indistinguishable in their ability to support embryonic development. The best overall efficiency (birth rate per oocytes injected) was obtained with frozen-thawed DBA/2 strain elongated spermatids (23.2+/-4.2%).
Conclusions/significance: The present study provides the first comprehensive information on ICSI using the mouse as a model and will contribute to the efficient use of materials, time, and efforts in biomedical research and clinics involving ICSI.
Conflict of interest statement
Figures
References
-
- Uehara T, Yanagimachi R. Microsurgical injection of spermatozoa into hamster eggs with subsequent transformation of sperm nuclei into male pronuclei. Biol Reprod. 1976;15:467–470. - PubMed
-
- Uehara T, Yanagimachi R. Behavior of nuclei of testicular, caput and cauda epididymal spermatozoa injected into hamster eggs. Biol Reprod. 1977;16:315–321. - PubMed
-
- Yanagimachi R. Intracytoplasmic injection of spermatozoa and spermatogenic cells: its biology and applications in humans and animals. Reprod Biomed Online. 2005;10:247–288. - PubMed
-
- Ogura A, Ogonuki N, Miki H, Inoue K. Microinsemination and nuclear transfer using male germ cells. Int Rev Cytol. 2005;246:189–229. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

