Microtubule depolymerization potentiates alpha-synuclein oligomerization
- PMID: 20552056
- PMCID: PMC2874407
- DOI: 10.3389/neuro.24.005.2009
Microtubule depolymerization potentiates alpha-synuclein oligomerization
Abstract
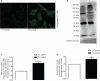
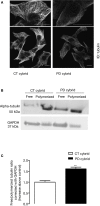
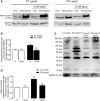
Parkinson's disease (PD) is associated with perturbed mitochondria function and alpha-synuclein fibrillization. We evaluated potential mechanistic links between mitochondrial dysfunction and alpha-synuclein aggregation. We studied a PD cytoplasmic hybrid (cybrid) cell line in which platelet mitochondria from a PD subject were transferred to NT2 neuronal cells previously depleted of endogenous mitochondrial DNA. Compared to a control cybrid cell line, the PD line showed reduced ATP levels, an increased free/polymerized tubulin ratio, and alpha-synuclein oligomer accumulation. Taxol (which stabilizes microtubules) normalized the PD tubulin ratio and reduced alpha-synuclein oligomerization. A nexus exists between mitochondrial function, cytoskeleton homeostasis, and alpha-synuclein oligomerization. In our model, mitochondrial dysfunction triggers an increased free tubulin, which destabilizes the microtubular network and promotes alpha-synuclein oligomerization.
Keywords: ATP; Parkinson disease; alpha-synuclein; cybrids; mitochondria; tubulin.
Figures
References
-
- Cassarino D. S., Fall C. P., Swerdlow R. H., Smith T. S., Halvorsen E. M., Miller S. W., Parks J. K., Parker W. D., Bennet J. P. (1997). Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson's disease. Biochim. Biophys. Acta 1362, 77–86 - PubMed
LinkOut - more resources
Full Text Sources

