PAMAM-camptothecin conjugate inhibits proliferation and induces nuclear fragmentation in colorectal carcinoma cells
- PMID: 20552256
- PMCID: PMC3092430
- DOI: 10.1007/s11095-010-0179-6
PAMAM-camptothecin conjugate inhibits proliferation and induces nuclear fragmentation in colorectal carcinoma cells
Abstract
Purpose: To synthesize and characterize a poly (amido amine) dendrimer-camptothecin (PAMAM-CPT) conjugate and evaluate its activity on human colorectal carcinoma cells (HCT-116).
Methods: The attachment of CPT to amine-terminated PAMAM was facilitated through a succinic acid-glycine linker. The conjugate was characterized for absence of small molecular weight impurities, size and drug content. Stability of the conjugate in PBS and growth media and its in vitro activity on HCT-116 were studied. Cell cycle arrest and nuclear fragmentation upon PAMAM-CPT treatment were investigated.
Results: The conjugate was stable under physiological pH (7.4) in PBS and in growth media (with 10% FBS) with minimal release of 4% and 6% drug, respectively, at 48 h. PAMAM-CPT inhibited proliferation of HCT-116 cells with an IC50 value of 1.6 ± 0.3 µM. The conjugate induced signs of cell cycle arrest with up to 68% of cells blocked in the G(2) phase. Confocal images of cells treated with PAMAM-CPT suggest nuclear fragmentation and formation of apoptotic bodies.
Conclusions: Results show that the PAMAM-CPT conjugate was active against colorectal cancer cells in vitro, inhibiting their growth and inducing nuclear fragmentation. Coupled with the ability to target macromolecular therapeutics to tumors, this conjugate shows promise for cancer chemotherapy.
Figures
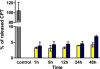
 ) and growth media (
) and growth media ( ). 115 μM of the conjugate was incubated at 37°C, and aliquots were drawn at various time points over 48 h. Samples were later analyzed by HPLC. Control measurement shows bar with 100% equivalent of free CPT. Mean values±S.D. from three independent experiments are presented.
). 115 μM of the conjugate was incubated at 37°C, and aliquots were drawn at various time points over 48 h. Samples were later analyzed by HPLC. Control measurement shows bar with 100% equivalent of free CPT. Mean values±S.D. from three independent experiments are presented.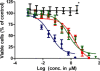
 PAMAM-CPT;
PAMAM-CPT;  S-G-CPT;
S-G-CPT;  CPT. Cells were incubated with various concentrations of drugs for 48 h. Relative cell number was determined by a WST-8 assay. Mean values±S.D. from three independent experiments are presented. * indicates PAMAM G4.0 polymer alone treatment at corresponding conjugate equivalent concentrations.
CPT. Cells were incubated with various concentrations of drugs for 48 h. Relative cell number was determined by a WST-8 assay. Mean values±S.D. from three independent experiments are presented. * indicates PAMAM G4.0 polymer alone treatment at corresponding conjugate equivalent concentrations.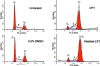
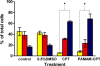
 S;
S;  G2. Cells were prepared, data acquired and analyzed as described in the legend of Fig. 3. Mean values±S.D. from three independent experiments are presented. Statistical comparisons between untreated and treated cells were performed. * indicates statistically significant data (p<0.05).
G2. Cells were prepared, data acquired and analyzed as described in the legend of Fig. 3. Mean values±S.D. from three independent experiments are presented. Statistical comparisons between untreated and treated cells were performed. * indicates statistically significant data (p<0.05).

Similar articles
-
Poly(amido amine) dendrimers as absorption enhancers for oral delivery of camptothecin.Int J Pharm. 2013 Nov 1;456(1):175-85. doi: 10.1016/j.ijpharm.2013.07.071. Epub 2013 Aug 8. Int J Pharm. 2013. PMID: 23933439 Free PMC article.
-
Enhanced anti-hepatocarcinoma efficacy by GLUT1 targeting and cellular microenvironment-responsive PAMAM-camptothecin conjugate.Drug Deliv. 2018 Nov;25(1):153-165. doi: 10.1080/10717544.2017.1419511. Drug Deliv. 2018. PMID: 29282992 Free PMC article.
-
Carboxyl-terminated PAMAM-SN38 conjugates: synthesis, characterization, and in vitro evaluation.Bioconjug Chem. 2010 Oct 20;21(10):1804-10. doi: 10.1021/bc100094z. Bioconjug Chem. 2010. PMID: 20836544 Free PMC article.
-
Charge-reversal polyamidoamine dendrimer for cascade nuclear drug delivery.Nanomedicine (Lond). 2010 Oct;5(8):1205-17. doi: 10.2217/nnm.10.86. Nanomedicine (Lond). 2010. PMID: 21039198
-
Antitumor activity of poly(ethylene glycol)-camptothecin conjugate: the inhibition of tumor growth in vivo.J Control Release. 2005 Dec 10;110(1):90-102. doi: 10.1016/j.jconrel.2005.09.050. Epub 2005 Nov 4. J Control Release. 2005. PMID: 16271793
Cited by
-
RNA Nanotechnology to Solubilize Hydrophobic Antitumor Drug for Targeted Delivery.Adv Sci (Weinh). 2019 Sep 30;6(22):1900951. doi: 10.1002/advs.201900951. eCollection 2019 Nov. Adv Sci (Weinh). 2019. PMID: 31763137 Free PMC article.
-
Cationic PAMAM dendrimers disrupt key platelet functions.Mol Pharm. 2012 Jun 4;9(6):1599-611. doi: 10.1021/mp2006054. Epub 2012 May 4. Mol Pharm. 2012. PMID: 22497592 Free PMC article.
-
Cytotoxicity of Dendrimers.Biomolecules. 2019 Aug 1;9(8):330. doi: 10.3390/biom9080330. Biomolecules. 2019. PMID: 31374911 Free PMC article. Review.
-
Intracellular Ca2+ release mediates cationic but not anionic poly(amidoamine) (PAMAM) dendrimer-induced tight junction modulation.Pharm Res. 2014 Sep;31(9):2429-38. doi: 10.1007/s11095-014-1338-y. Epub 2014 Mar 20. Pharm Res. 2014. PMID: 24648136
-
Endosomal pH, Redox Dual-Sensitive Prodrug Micelles Based on Hyaluronic Acid for Intracellular Camptothecin Delivery and Active Tumor Targeting in Cancer Therapy.Pharmaceutics. 2024 Oct 14;16(10):1327. doi: 10.3390/pharmaceutics16101327. Pharmaceutics. 2024. PMID: 39458656 Free PMC article.
References
-
- Ulukan H, Swaan PW. Camptothecins: a review of their chemotherapeutic potential. Drugs. 2002;62:2039–57. - PubMed
-
- Li QY, Zu YG, Shi RZ, Yao LP. Review camptothecin: current perspectives. Curr Med Chem. 2006;13:2021–39. - PubMed
-
- Hatefi A, Amsden B. Camptothecin delivery methods. Pharm Res. 2002;19:1389–99. - PubMed
-
- Facts about cancer deaths. [accessed 08/31 2009]. http://www.who.int/mediacentre/factsheets/fs297/en/, part of World Health Organization http://www.who.int/en/
-
- Current treatment options for colon cancer. [accessed 08/31 2009]. http://www.cancer.gov/cancertopics/pdq/treatment/colon/Patient/page4, part of National Cancer Institute http://www.cancer.gov/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

