Magnetic resonance imaging of brain angiogenesis after stroke
- PMID: 20552268
- PMCID: PMC2911530
- DOI: 10.1007/s10456-010-9174-0
Magnetic resonance imaging of brain angiogenesis after stroke
Abstract
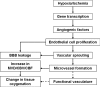
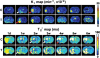
Stroke is a major cause of mortality and long-term disability worldwide. The initial changes in local perfusion and tissue status underlying loss of brain function are increasingly investigated with noninvasive imaging methods. In addition, there is a growing interest in imaging of processes that contribute to post-stroke recovery. In this review, we discuss the application of magnetic resonance imaging (MRI) to assess the formation of new vessels by angiogenesis, which is hypothesized to participate in brain plasticity and functional recovery after stroke. The excellent soft tissue contrast, high spatial and temporal resolution, and versatility render MRI particularly suitable to monitor the dynamic processes involved in vascular remodeling after stroke. Here we review recent advances in the field of MR imaging that are aimed at assessment of tissue perfusion and microvascular characteristics, including cerebral blood flow and volume, vascular density, size and integrity. The potential of MRI to noninvasively monitor the evolution of post-ischemic angiogenic processes is demonstrated from a variety of in vivo studies in experimental stroke models. Finally, we discuss some pitfalls and limitations that may critically affect the accuracy and interpretation of MRI-based measures of (neo)vascularization after stroke.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

