FLPe functions in zebrafish embryos
- PMID: 20552273
- PMCID: PMC3051101
- DOI: 10.1007/s11248-010-9410-9
FLPe functions in zebrafish embryos
Abstract
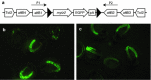
To assay the efficiency of the FLP/FRT site-specific recombination system in Danio rerio, a construct consisting of a muscle-specific promoter driving EGFP flanked by FRT sites was developed. FLPe capped RNA was microinjected into transgenic single cell stage zebrafish embryos obtained by crossing hemizygous transgenic males with wild-type females. By 48 h post fertilization (hpf), the proportion of embryos displaying green fluorescence following FLPe RNA microinjection was significantly lower (7.7%; P < 0.001) than would be expected from a cross in the absence of the recombinase (50%). Embryos that retained fluorescence displayed marked mosaicism. Inheritance of the excised transgene in non-fluorescent, transgenic embryos was verified by PCR analysis and FLPe-mediated recombination was confirmed by DNA sequencing. Sperm derived from confirmed transgenic males in these experiments was used to fertilize wild-type eggs to determine whether germline excision of the transgene had occurred. Clutches sired by FLPe-microinjected males contained 0-4% fluorescent embryos. Transgenic males that were phenotypically wild-type produced no fluorescent progeny, demonstrating complete excision of the transgene from their germline. FLPe microinjected males that retained some fluorescent muscle expression produced a small proportion of fluorescent offspring, suggesting that in mosaic males not all germline cells had undergone FLPe-mediated transgene excision. Our results show that FLPe, which is derived from Saccharomyces cerevisiae, is an efficient recombinase in zebrafish maintained at 28.5°C.
Figures

Similar articles
-
FLP recombinase-mediated site-specific recombination in silkworm, Bombyx mori.PLoS One. 2012;7(6):e40150. doi: 10.1371/journal.pone.0040150. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768245 Free PMC article.
-
Improved FLP recombinase, FLPe, efficiently removes marker gene from transgene locus developed by Cre-lox mediated site-specific gene integration in rice.Mol Biotechnol. 2011 Sep;49(1):82-9. doi: 10.1007/s12033-011-9381-y. Mol Biotechnol. 2011. PMID: 21274659
-
Highly efficient and inducible DNA excision in transgenic silkworms using the FLP/FRT site-specific recombination system.Transgenic Res. 2016 Dec;25(6):795-811. doi: 10.1007/s11248-016-9970-4. Epub 2016 Jun 22. Transgenic Res. 2016. PMID: 27334499
-
Transgenic fish systems and their application in ecotoxicology.Crit Rev Toxicol. 2015 Feb;45(2):124-41. doi: 10.3109/10408444.2014.965805. Epub 2014 Nov 14. Crit Rev Toxicol. 2015. PMID: 25394772 Review.
-
FLPing Genes On and Off in Drosophila.Methods Mol Biol. 2017;1642:195-209. doi: 10.1007/978-1-4939-7169-5_13. Methods Mol Biol. 2017. PMID: 28815502 Free PMC article. Review.
Cited by
-
One-step efficient generation of dual-function conditional knockout and geno-tagging alleles in zebrafish.Elife. 2019 Oct 30;8:e48081. doi: 10.7554/eLife.48081. Elife. 2019. PMID: 31663848 Free PMC article.
-
A noninvasive photoactivatable split-Cre recombinase system for genome engineering in zebrafish.iScience. 2024 Jul 8;27(8):110476. doi: 10.1016/j.isci.2024.110476. eCollection 2024 Aug 16. iScience. 2024. PMID: 39129833 Free PMC article.
-
FLP recombinase-mediated site-specific recombination in silkworm, Bombyx mori.PLoS One. 2012;7(6):e40150. doi: 10.1371/journal.pone.0040150. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768245 Free PMC article.
-
Genetics of photoreceptor degeneration and regeneration in zebrafish.Cell Mol Life Sci. 2011 Feb;68(4):651-9. doi: 10.1007/s00018-010-0563-8. Epub 2010 Oct 24. Cell Mol Life Sci. 2011. PMID: 20972813 Free PMC article. Review.
-
Learning to Fish with Genetics: A Primer on the Vertebrate Model Danio rerio.Genetics. 2016 Jul;203(3):1069-89. doi: 10.1534/genetics.116.190843. Genetics. 2016. PMID: 27384027 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

