Molecular imaging of inflammation and intraplaque vasa vasorum: a step forward to identification of vulnerable plaques?
- PMID: 20552308
- PMCID: PMC2940038
- DOI: 10.1007/s12350-010-9263-x
Molecular imaging of inflammation and intraplaque vasa vasorum: a step forward to identification of vulnerable plaques?
Abstract
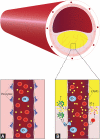
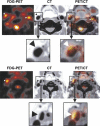
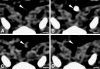
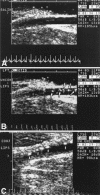
Current developments in cardiovascular biology and imaging enable the noninvasive molecular evaluation of atherosclerotic vascular disease. Intraplaque neovascularization sprouting from the adventitial vasa vasorum has been identified as an independent predictor of intraplaque hemorrhage and plaque rupture. These intraplaque vasa vasorum result from angiogenesis, most likely under influence of hypoxic and inflammatory stimuli. Several molecular imaging techniques are currently available. Most experience has been obtained with molecular imaging using positron emission tomography and single photon emission computed tomography. Recently, the development of targeted contrast agents has allowed molecular imaging with magnetic resonance imaging, ultrasound and computed tomography. The present review discusses the use of these molecular imaging techniques to identify inflammation and intraplaque vasa vasorum to identify vulnerable atherosclerotic plaques at risk of rupture and thrombosis. The available literature on molecular imaging techniques and molecular targets associated with inflammation and angiogenesis is discussed, and the clinical applications of molecular cardiovascular imaging and the use of molecular techniques for local drug delivery are addressed.
Figures

References
-
- Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. - PubMed
-
- Kastelein JJP, de Groot E. Ultrasound imaging techniques for the evaluation of cardiovascular therapies. Eur Heart J. 2008;29:849–858. - PubMed
-
- Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. - PubMed
-
- Barger AC, Beeuwkes R, Lainey LL, Silverman KJ. Hypothesis: Vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

