Increase in surface hydrophobicity of the cataract-associated P23T mutant of human gammaD-crystallin is responsible for its dramatically lower, retrograde solubility
- PMID: 20553008
- PMCID: PMC2913551
- DOI: 10.1021/bi100664s
Increase in surface hydrophobicity of the cataract-associated P23T mutant of human gammaD-crystallin is responsible for its dramatically lower, retrograde solubility
Abstract
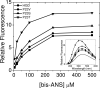
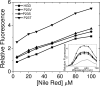
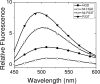
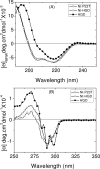
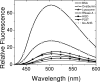
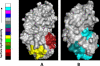
The cataract-associated Pro23 to Thr (P23T) mutation in human gammaD-crystallin (HGD) has a variety of phenotypes and is geographically widespread. Therefore, there is considerable interest in understanding the molecular basis of cataract formation due to this mutation. We showed earlier [Pande, A., et al. (2005) Biochemistry 44, 2491-2500] that the probable basis of opacity in this case is the severely compromised, retrograde solubility and aggregation of P23T relative to HGD. The dramatic solubility change occurs even as the structure of the mutant protein remains essentially unchanged in vitro. We proposed that the retrograde solubility and aggregation of P23T were mediated by net hydrophobic, protein-protein interactions. On the basis of these initial findings for P23T and related mutants, and the subsequent finding that they show atypical phase behavior [McManus, J. J., et al. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 16856-16861], we concluded that the protein clusters formed in solutions of the mutant proteins were held together by net hydrophobic, anisotropic interactions. Here we show, using chemical probes, that the surface hydrophobicities of these mutants are inversely related to their solubility. Furthermore, by probing the isolated N-terminal domains of HGD and P23T directly, we find that the increase in the surface hydrophobicity of P23T is localized in the N-terminal domain. Modeling studies suggest the presence of sticky patches on the surface of the N-terminal domain that could be engaged in the formation of protein clusters via hydrophobic protein-protein interactions. This work thus provides direct evidence of the dominant role played by net hydrophobic and anisotropic protein-protein interactions in the aggregation of P23T.
Figures

Similar articles
-
NMR study of the cataract-linked P23T mutant of human gammaD-crystallin shows minor changes in hydrophobic patches that reflect its retrograde solubility.Biochem Biophys Res Commun. 2009 Apr 24;382(1):196-9. doi: 10.1016/j.bbrc.2009.03.007. Epub 2009 Mar 9. Biochem Biophys Res Commun. 2009. PMID: 19275895 Free PMC article.
-
Increased hydrophobicity and decreased backbone flexibility explain the lower solubility of a cataract-linked mutant of γD-crystallin.J Mol Biol. 2011 Sep 30;412(4):647-59. doi: 10.1016/j.jmb.2011.07.058. Epub 2011 Jul 30. J Mol Biol. 2011. PMID: 21827768 Free PMC article.
-
Decrease in protein solubility and cataract formation caused by the Pro23 to Thr mutation in human gamma D-crystallin.Biochemistry. 2005 Feb 22;44(7):2491-500. doi: 10.1021/bi0479611. Biochemistry. 2005. PMID: 15709761
-
The P23T cataract mutation causes loss of solubility of folded gammaD-crystallin.J Mol Biol. 2004 Oct 15;343(2):435-44. doi: 10.1016/j.jmb.2004.08.050. J Mol Biol. 2004. PMID: 15451671
-
Aggregation inhibitory effect of vitamin C on cataract-associated P23T γD-crystallin.Int J Biol Macromol. 2025 Apr;302:140579. doi: 10.1016/j.ijbiomac.2025.140579. Epub 2025 Feb 1. Int J Biol Macromol. 2025. PMID: 39900151
Cited by
-
Mutations and mechanisms in congenital and age-related cataracts.Exp Eye Res. 2017 Mar;156:95-102. doi: 10.1016/j.exer.2016.06.011. Epub 2016 Jun 19. Exp Eye Res. 2017. PMID: 27334249 Free PMC article. Review.
-
Cataract-Associated New Mutants S175G/H181Q of βΒ2-Crystallin and P24S/S31G of γD-Crystallin Are Involved in Protein Aggregation by Structural Changes.Int J Mol Sci. 2020 Sep 5;21(18):6504. doi: 10.3390/ijms21186504. Int J Mol Sci. 2020. PMID: 32899552 Free PMC article.
-
The Y46D Mutation Destabilizes Dense Packing of the Second Greek Key Pair of Human γC-Crystallin Causing Congenital Nuclear Cataracts.Biochemistry. 2023 Jun 20;62(12):1864-1877. doi: 10.1021/acs.biochem.2c00628. Epub 2023 May 15. Biochemistry. 2023. PMID: 37184593 Free PMC article.
-
Cataract-causing defect of a mutant γ-crystallin proceeds through an aggregation pathway which bypasses recognition by the α-crystallin chaperone.PLoS One. 2012;7(5):e37256. doi: 10.1371/journal.pone.0037256. Epub 2012 May 24. PLoS One. 2012. PMID: 22655036 Free PMC article.
-
Human cataract mutations in EPHA2 SAM domain alter receptor stability and function.PLoS One. 2012;7(5):e36564. doi: 10.1371/journal.pone.0036564. Epub 2012 May 3. PLoS One. 2012. PMID: 22570727 Free PMC article.
References
-
- Shentu X, Yao K, Xu W, Zheng S, Hu S, Gong X. Special fasciculiform cataract caused by a mutation in the gammaD-crystallin gene. Mol Vis. 2004;10:233–239. - PubMed
-
- Xu WZ, Zheng S, Xu SJ, Huang W, Yao K, Zhang SZ. Autosomal dominant coralliform cataract related to a missense mutation of the gammaD-crystallin gene. Chin Med J (Engl) 2004;117:727–732. - PubMed
-
- Mackay DS, Andley UP, Shiels A. A missense mutation in the gammaD crystallin gene (CRYGD) associated with autosomal dominant “coral-like” cataract linked to chromosome 2q. Mol Vis. 2004;10:155–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

