Atorvastatin or transgenic expression of TFPI inhibits coagulation initiated by anti-nonGal IgG binding to porcine aortic endothelial cells
- PMID: 20553382
- PMCID: PMC2965779
- DOI: 10.1111/j.1538-7836.2010.03950.x
Atorvastatin or transgenic expression of TFPI inhibits coagulation initiated by anti-nonGal IgG binding to porcine aortic endothelial cells
Abstract
Background: Intravascular thrombosis remains a barrier to successful xenotransplantation. Tissue factor (TF) expression on porcine aortic endothelial cells (PAECs), which results from their activation by xenoreactive antibodies (Abs) to Galα1,3Gal (Gal) and subsequent complement activation, plays an important role.
Objectives: The present study aimed to clarify the role of Abs directed against nonGal antigens in the activation of PAECs to express functional TF and to investigate selected methods of inhibiting TF activity.
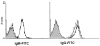
Methods: PAECs from wild-type (WT), α1,3-galactosyltransferase gene-knockout (GT-KO) pigs, or pigs transgenic for CD46 or tissue factor pathway inhibitor (TFPI), were incubated with naïve baboon serum (BS) or sensitized BS (with high anti-nonGal Ab levels). TF activity of PAECs was assessed.
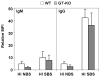
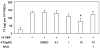
Results: Only fresh, but not heat-inactivated (HI), naïve BS activated WT PAECs to express functional TF. Similarly, PAECs from CD46 pigs were resistant to activation by naïve BS, but not to activation by fresh or HI sensitized BS. HI sensitized BS also activated GT-KO PAECs to induce TF activity. TF expression on PAECs induced by anti-nonGal Abs was inhibited if serum was pretreated with (i) an anti-IgG Fab Ab or (ii) atorvastatin, or (iii) when PAECs were transgenic for TFPI.
Conclusions: Anti-nonGal IgG Abs activated PAECs to induce TF activity through a complement-independent pathway. This implies that GT-KO pigs expressing a complement-regulatory protein may be insufficient to prevent the activation of PAECs. Genetic modification with an 'anticoagulant' gene (e.g. TFPI) or a therapeutic approach (e.g. atorvastatin) will be required to prevent coagulation dysregulation after pig-to-primate organ transplantation.
© 2010 International Society on Thrombosis and Haemostasis.
Figures
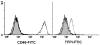
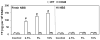
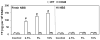








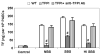
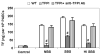


References
-
- Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–47. - PubMed
-
- Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, Mueller N, Yamada K, Greenstein JL, Hawley RJ, Patience C, Awwad M, Fishman JA, Robson SC, Schuurman HJ, Sachs DH, Cooper DK. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. - PubMed
-
- Nagayasu T, Saadi S, Holzknecht RA, Plummer TB, Platt JL. Expression of tissue factor mRNA in cardiac xenografts: clues to the pathogenesis of acute vascular rejection. Transplantation. 2000;69:475–82. - PubMed
-
- Gollackner B, Goh SK, Qawi I, Buhler L, Knosalla C, Daniel S, Kaczmarek E, Awwad M, Cooper DK, Robson SC. Acute vascular rejection of xenografts: roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation. 2004;77:1735–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

