Confocal analysis of nervous system architecture in direct-developing juveniles of Neanthes arenaceodentata (Annelida, Nereididae)
- PMID: 20553614
- PMCID: PMC2909921
- DOI: 10.1186/1742-9994-7-17
Confocal analysis of nervous system architecture in direct-developing juveniles of Neanthes arenaceodentata (Annelida, Nereididae)
Abstract
Background: Members of Family Nereididae have complex neural morphology exemplary of errant polychaetes and are leading research models in the investigation of annelid nervous systems. However, few studies focus on the development of their nervous system morphology. Such data are particularly relevant today, as nereidids are the subjects of a growing body of "evo-devo" work concerning bilaterian nervous systems, and detailed knowledge of their developing neuroanatomy facilitates the interpretation of gene expression analyses. In addition, new data are needed to resolve discrepancies between classic studies of nereidid neuroanatomy. We present a neuroanatomical overview based on acetylated alpha-tubulin labeling and confocal microscopy for post-embryonic stages of Neanthes arenaceodentata, a direct-developing nereidid.
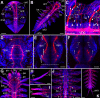
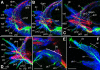
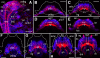
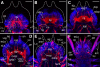
Results: At hatching (2-3 chaetigers), the nervous system has developed much of the complexity of the adult (large brain, circumesophageal connectives, nerve cords, segmental nerves), and the stomatogastric nervous system is partially formed. By the 5-chaetiger stage, the cephalic appendages and anal cirri are well innervated and have clear connections to the central nervous system. Within one week of hatching (9-chaetigers), cephalic sensory structures (e.g., nuchal organs, Langdon's organs) and brain substructures (e.g., corpora pedunculata, stomatogastric ganglia) are clearly differentiated. Additionally, the segmental-nerve architecture (including interconnections) matches descriptions of other, adult nereidids, and the pharynx has developed longitudinal nerves, nerve rings, and ganglia. All central roots of the stomatogastric nervous system are distinguishable in 12-chaetiger juveniles. Evidence was also found for two previously undescribed peripheral nerve interconnections and aspects of parapodial muscle innervation.
Conclusions: N. arenaceodentata has apparently lost all essential trochophore characteristics typical of nereidids. Relative to the polychaete Capitella, brain separation from a distinct epidermis occurs later in N. arenaceodentata, indicating different mechanisms of prostomial development. Our observations of parapodial innervation and the absence of lateral nerves in N. arenaceodentata are similar to a 19th century study of Alitta virens (formerly Nereis/Neanthes virens) but contrast with a more recent study that describes a single parapodial nerve pattern and lateral nerve presence in A. virens and two other genera. The latter study apparently does not account for among-nereidid variation in these major neural features.
Figures

Similar articles
-
Expression of the Lhx genes apterous and lim1 in an errant polychaete: implications for bilaterian appendage evolution, neural development, and muscle diversification.Evodevo. 2013 Feb 1;4(1):4. doi: 10.1186/2041-9139-4-4. Evodevo. 2013. PMID: 23369627 Free PMC article.
-
Expression of Distal-less, dachshund, and optomotor blind in Neanthes arenaceodentata (Annelida, Nereididae) does not support homology of appendage-forming mechanisms across the Bilateria.Dev Genes Evol. 2010 Dec;220(9-10):275-95. doi: 10.1007/s00427-010-0346-0. Epub 2010 Nov 30. Dev Genes Evol. 2010. PMID: 21116826 Free PMC article.
-
Structure of the nervous system of Myzostoma cirriferum (Annelida) as revealed by immunohistochemistry and cLSM analyses.J Morphol. 2000 Aug;245(2):87-98. doi: 10.1002/1097-4687(200008)245:2<87::AID-JMOR1>3.0.CO;2-W. J Morphol. 2000. PMID: 10906744
-
A remarkable new deep-sea nereidid (Annelida: Nereididae) with gills.PLoS One. 2024 Mar 6;19(3):e0297961. doi: 10.1371/journal.pone.0297961. eCollection 2024. PLoS One. 2024. PMID: 38446781 Free PMC article. Review.
-
The Nereid on the rise: Platynereis as a model system.Evodevo. 2021 Sep 27;12(1):10. doi: 10.1186/s13227-021-00180-3. Evodevo. 2021. PMID: 34579780 Free PMC article. Review.
Cited by
-
Mass Start or Time Trial? Structure of the Nervous System and Neuroregeneration in Pygospio elegans (Spionidae, Annelida).Biology (Basel). 2023 Nov 9;12(11):1412. doi: 10.3390/biology12111412. Biology (Basel). 2023. PMID: 37998011 Free PMC article.
-
Histamine and gamma-aminobutyric acid in the nervous system of Pygospio elegans (Annelida: Spionidae): structure and recovery during reparative regeneration.BMC Zool. 2022 Dec 7;7(1):58. doi: 10.1186/s40850-022-00160-7. BMC Zool. 2022. PMID: 37170300 Free PMC article.
-
Owenia fusiformis - a basally branching annelid suitable for studying ancestral features of annelid neural development.BMC Evol Biol. 2016 Jun 16;16(1):129. doi: 10.1186/s12862-016-0690-4. BMC Evol Biol. 2016. PMID: 27306767 Free PMC article.
-
Immunohistochemical and ultrastructural properties of the larval ciliary band-associated strand in the sea urchin Hemicentrotus pulcherrimus.Front Zool. 2016 Jun 16;13:27. doi: 10.1186/s12983-016-0159-8. eCollection 2016. Front Zool. 2016. PMID: 27313654 Free PMC article.
-
May the palps be with you - new insights into the evolutionary origin of anterior appendages in Terebelliformia (Annelida).BMC Zool. 2021 Nov 16;6(1):30. doi: 10.1186/s40850-021-00094-6. BMC Zool. 2021. PMID: 37170288 Free PMC article.
References
-
- de Quatrefages A. Sur le système nerveux des annélides. Ann Sci Nat Sér 3 Zool. 1844;2:81–104.
-
- de Quatrefages A. Études sur les types inférieures de l'embranchement des annelés. Ann Sci Nat Sér 3 Zool. 1850;14:329–398.
-
- Bullock TH, Horridge GA. Structure and Function in the Nervous System of Invertebrates. Vol. 1. San Francisco: WH Freeman and Co; 1965.
-
- Dorsett DA. In: Physiology of Annelids. Mill PJ, editor. London: Academic Press; 1978. Organization of the nerve cord; pp. 115–160.
-
- Dhainaut-Courtois N, Golding DW. In: The Ultrastructure of Polychaeta. Microfauna Marina 4. Westheide W, Hermans CO, editor. Stuttgart: Gustav Fischer Verlag; 1988. Nervous system; pp. 89–110.

