Development of a PROMIS item bank to measure pain interference
- PMID: 20554116
- PMCID: PMC2916053
- DOI: 10.1016/j.pain.2010.04.025
Development of a PROMIS item bank to measure pain interference
Abstract
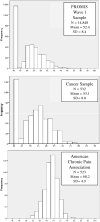
This paper describes the psychometric properties of the PROMIS-pain interference (PROMIS-PI) bank. An initial candidate item pool (n=644) was developed and evaluated based on the review of existing instruments, interviews with patients, and consultation with pain experts. From this pool, a candidate item bank of 56 items was selected and responses to the items were collected from large community and clinical samples. A total of 14,848 participants responded to all or a subset of candidate items. The responses were calibrated using an item response theory (IRT) model. A final 41-item bank was evaluated with respect to IRT assumptions, model fit, differential item function (DIF), precision, and construct and concurrent validity. Items of the revised bank had good fit to the IRT model (CFI and NNFI/TLI ranged from 0.974 to 0.997), and the data were strongly unidimensional (e.g., ratio of first and second eigenvalue=35). Nine items exhibited statistically significant DIF. However, adjusting for DIF had little practical impact on score estimates and the items were retained without modifying scoring. Scores provided substantial information across levels of pain; for scores in the T-score range 50-80, the reliability was equivalent to 0.96-0.99. Patterns of correlations with other health outcomes supported the construct validity of the item bank. The scores discriminated among persons with different numbers of chronic conditions, disabling conditions, levels of self-reported health, and pain intensity (p<0.0001). The results indicated that the PROMIS-PI items constitute a psychometrically sound bank. Computerized adaptive testing and short forms are available.
Copyright 2010 International Association for the Study of Pain. All rights reserved.
Conflict of interest statement
Figures
References
-
- Anastasi A. Psychological Testing. New York: Macmillan Publishing Company; 1988.
-
- Becker J, Schwartz C, Saris-Baglama RN, Kosinski M, Bjorner JB. Using item response theory (IRT) for developing and evaluating the Pain Impact Questionnaire (PIQ-6TM) Pain Med. 2007. pp. S129–S144. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2662709.
-
- Bentler P. Comparative fit indices in structural models. Psycho Bull. 1990;107:238–46. - PubMed
-
- Bernstein IH, Jaremko ME, Hinkley BS. On the utility of the West Haven-Yale Multidimensional Pain Inventory. Spine. 1995. pp. 956–63. http://www.ncbi.nlm.nih.gov/pubmed/7644962. - PubMed
-
- Bjorner JB, Smith KJ, Stone C, Sun X. Lincoln, RI: QualityMetric; 2007. IRTFIT: A Macro for Item Fit and Local Dependence Tests under IRT Models. http://outcomes.cancer.gov/areas/measurement/irt_model_fit.html.
Publication types
MeSH terms
Grants and funding
- U01AR52155/AR/NIAMS NIH HHS/United States
- U01 AR052170/AR/NIAMS NIH HHS/United States
- U01 AR052181/AR/NIAMS NIH HHS/United States
- U01AR52170/AR/NIAMS NIH HHS/United States
- U01AR52158/AR/NIAMS NIH HHS/United States
- U01AR52171/AR/NIAMS NIH HHS/United States
- U01 AR052155/AR/NIAMS NIH HHS/United States
- U01AR52186/AR/NIAMS NIH HHS/United States
- U01 AR052171/AR/NIAMS NIH HHS/United States
- U01 AR052186/AR/NIAMS NIH HHS/United States
- U01 AR052177/AR/NIAMS NIH HHS/United States
- U01AR52177/AR/NIAMS NIH HHS/United States
- U01AR52181/AR/NIAMS NIH HHS/United States
- U01 AR052158/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous