Enhanced effect of soluble transforming growth factor-beta receptor II and IFN-gamma fusion protein in reversing hepatic fibrosis
- PMID: 20554496
- PMCID: PMC3474166
- DOI: 10.1186/2047-783x-15-4-152
Enhanced effect of soluble transforming growth factor-beta receptor II and IFN-gamma fusion protein in reversing hepatic fibrosis
Abstract
Objective: To examine the in vivo anti-fibrotic effect of rat soluble transforming growth factor beta receptor II (RsTbetaRII) and IFN-gamma fusion protein (RsTbRII-IFN-gamma) in rat hepatic fibrosis model.
Methods: Model rats were divided into five groups and treated i.m. for 8 weeks: 1) fibrotic model group (each rat, 100 microl of 0.9% NaCl day superset-1); 2) RsTbetaRII-IFN-gamma treatment group (each rat, 0.136 mg x day(-1); 3) IFN-gamma treatment group (each rat, 7.5 MU x day(-1); 4) RsTbetaRII treatment group (each rat, 0.048 mg x day(-1); and 5) mixture of IFN-gamma and RsTbetaRII treatment group (each rat, IFN-gamma 7.5 MU x day(-1)+ RsTbetaRII 0.048 mg x day(-1). After treatment, hepatic fibrogenesis was evaluated by histopathological analysis and measurement of collagen III, alpha-smooth muscle actin (alpha-SMA), TGF-beta1, TGF-betaRII and their mRNA.
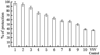
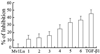
Results: Immunohistochemistry, Western blot and real-time RT-PCR showed that RsTbetaRII-IFN-gamma treatment significantly inhibited liver expression of collagen III, alpha-SMA, TGF-beta1 and TGF-betaRII at both protein and mRNA levels. Histopathological analysis also showed that the enhanced anti-fibrotic effects were achieved in model rats treated with RsTbetaRII-IFN-gamma.
Conclusion: Our results confirmed that RsTbetaRII-IFN-gamma has the enhanced effects in reversing hepatic fibrosis.
Figures
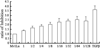






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

