Neural control of behavioural choice in juvenile crayfish
- PMID: 20554556
- PMCID: PMC2982233
- DOI: 10.1098/rspb.2010.1000
Neural control of behavioural choice in juvenile crayfish
Abstract
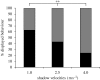
Natural selection leads to behavioural choices that increase the animal's fitness. The neuronal mechanisms underlying behavioural choice are still elusive and empirical evidence connecting neural circuit activation to adaptive behavioural output is sparse. We exposed foraging juvenile crayfish to approaching shadows of different velocities and found that slow-moving shadows predominantly activated a pair of giant interneurons, which mediate tail-flips that thrust the animals backwards and away from the approaching threat. Tail-flips also moved the animals farther away from an expected food source, and crayfish defaulted to freezing behaviour when faced with fast-approaching shadows. Under these conditions, tail-flipping, an ineffective and costly escape strategy was suppressed in favour of freezing, a more beneficial choice. The decision to freeze also dominated in the presence of a more desirable resource; however, the increased incentive was less effective in suppressing tail-flipping when paired with slow-moving visual stimuli that reliably evoked tail-flips in most animals. Together this suggests that crayfish make value-based decisions by weighing the costs and benefits of different behavioural options, and they select adaptive behavioural output based on the activation patterns of identifiable neural circuits.
Figures


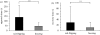

References
-
- Bergman D. A., Moore P. A.2003Field observations of intraspecific agonistic behaviour of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol. Bull. 205, 26–35 - PubMed
-
- Blanchard D. C., Griebel G., Blanchard R. J.2001Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci. Biobehav. Rev. 25, 205–218 (doi:10.1016/S0149-7634(01)00009-4) - DOI - PubMed
-
- Bowerman R. F., Larimer J. L.1974Command fibres in the circumoesophageal connectives of crayfish. I. Tonic fibres. J. Exp. Biol. 60, 95–117
-
- Briggman K. L., Abarbanel H. D. I., Kristan W. B., Jr2005Optical imaging of neuronal populations during decision-making. Science 307, 896–901 (doi:10.1126/science.1103736) - DOI - PubMed
-
- Calabrese R.2003Behavioral choices: how neuronal networks make decisions. Curr. Biol. 13, 140–142 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

