Topographic mapping--the olfactory system
- PMID: 20554703
- PMCID: PMC2908763
- DOI: 10.1101/cshperspect.a001776
Topographic mapping--the olfactory system
Abstract
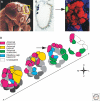
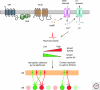
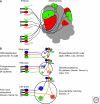
Sensory systems must map accurate representations of the external world in the brain. Although the physical senses of touch and vision build topographic representations of the spatial coordinates of the body and the field of view, the chemical sense of olfaction maps discontinuous features of chemical space, comprising an extremely large number of possible odor stimuli. In both mammals and insects, olfactory circuits are wired according to the convergence of axons from sensory neurons expressing the same odorant receptor. Synapses are organized into distinctive spherical neuropils--the olfactory glomeruli--that connect sensory input with output neurons and local modulatory interneurons. Although there is a strong conservation of form in the olfactory maps of mammals and insects, they arise using divergent mechanisms. Olfactory glomeruli provide a unique solution to the problem of mapping discontinuous chemical space onto the brain.
Figures


Similar articles
-
The olfactory bulb: coding and processing of odor molecule information.Science. 1999 Oct 22;286(5440):711-5. doi: 10.1126/science.286.5440.711. Science. 1999. PMID: 10531048 Review.
-
Molecular, anatomical, and functional organization of the Drosophila olfactory system.Curr Biol. 2005 Sep 6;15(17):1535-47. doi: 10.1016/j.cub.2005.07.034. Curr Biol. 2005. PMID: 16139208
-
Olfactory maps and odor images.Curr Opin Neurobiol. 2002 Aug;12(4):387-92. doi: 10.1016/s0959-4388(02)00348-3. Curr Opin Neurobiol. 2002. PMID: 12139985 Review.
-
The molecular logic of olfaction in Drosophila.Chem Senses. 2001 Feb;26(2):207-13. doi: 10.1093/chemse/26.2.207. Chem Senses. 2001. PMID: 11238253
-
Olfactory coding: non-linear amplification separates smells.Curr Biol. 2008 Jan 8;18(1):R29-32. doi: 10.1016/j.cub.2007.10.063. Curr Biol. 2008. PMID: 18177710
Cited by
-
All-Optical Volumetric Physiology for Connectomics in Dense Neuronal Structures.iScience. 2019 Dec 20;22:133-146. doi: 10.1016/j.isci.2019.11.011. Epub 2019 Nov 9. iScience. 2019. PMID: 31765994 Free PMC article.
-
The Smell of Blue Light: A New Approach toward Understanding an Olfactory Neuronal Network.Front Neurosci. 2011 May 23;5:72. doi: 10.3389/fnins.2011.00072. eCollection 2011. Front Neurosci. 2011. PMID: 21647413 Free PMC article.
-
Pheromone Perception in Fish: Mechanisms and Modulation by Internal Status.Integr Comp Biol. 2023 Aug 23;63(2):407-427. doi: 10.1093/icb/icad049. Integr Comp Biol. 2023. PMID: 37263784 Free PMC article. Review.
-
Conserved neural dynamics and computations across species in olfaction.bioRxiv [Preprint]. 2023 Apr 24:2023.04.24.538157. doi: 10.1101/2023.04.24.538157. bioRxiv. 2023. PMID: 37162844 Free PMC article. Preprint.
-
On the Fila Olfactoria and the Cribriform Region of the Crocodylia.J Morphol. 2025 Feb;286(2):e70036. doi: 10.1002/jmor.70036. J Morphol. 2025. PMID: 39985331 Free PMC article.
References
-
- Alenius M, Bohm S 1997. Identification of a novel neural cell adhesion molecule-related gene with a potential role in selective axonal projection. J Biol Chem 272: 26083–26086 - PubMed
-
- Axel R 1995. The molecular logic of smell. Sci Am 273: 154–159 - PubMed
-
- Barnea G, O’Donnell S, Mancia F, Sun X, Nemes A, Mendelsohn M, Axel R 2004. Odorant receptors on axon termini in the brain. Science 304: 1468. - PubMed
-
- Belluscio L, Gold GH, Nemes A, Axel R 1998. Mice deficient in G(olf) are anosmic. Neuron 20: 69–81 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
