Phosphatidic acid induces ligand-independent epidermal growth factor receptor endocytic traffic through PDE4 activation
- PMID: 20554760
- PMCID: PMC2921116
- DOI: 10.1091/mbc.E10-02-0167
Phosphatidic acid induces ligand-independent epidermal growth factor receptor endocytic traffic through PDE4 activation
Abstract
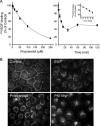
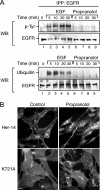
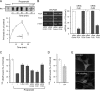
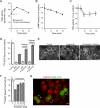
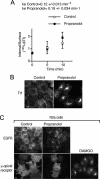
Endocytosis modulates EGFR function by compartmentalizing and attenuating or enhancing its ligand-induced signaling. Here we show that it can also control the cell surface versus intracellular distribution of empty/inactive EGFR. Our previous observation that PKA inhibitors induce EGFR internalization prompted us to test phosphatidic acid (PA) generated by phospholipase D (PLD) as an endogenous down-regulator of PKA activity, which activates rolipram-sensitive type 4 phosphodiesterases (PDE4) that degrade cAMP. We found that inhibition of PA hydrolysis by propranolol, in the absence of ligand, provokes internalization of inactive (neither tyrosine-phosphorylated nor ubiquitinated) EGFR, accompanied by a transient increase in PA levels and PDE4s activity. This EGFR internalization is mimicked by PA micelles and is strongly counteracted by PLD2 silencing, rolipram or forskolin treatment, and PKA overexpression. Accelerated EGFR endocytosis seems to be mediated by clathrin-dependent and -independent pathways, leading to receptor accumulation in juxtanuclear recycling endosomes, also due to a decreased recycling. Internalized EGFR can remain intracellular without degradation for several hours or return rapidly to the cell surface upon discontinuation of the stimulus. This novel regulatory mechanism of EGFR, also novel function of signaling PA, can transmodulate receptor accessibility in response to heterologous stimuli.
Figures


Similar articles
-
Phosphatidic acid-PKA signaling regulates p38 and ERK1/2 functions in ligand-independent EGFR endocytosis.Traffic. 2021 Oct;22(10):345-361. doi: 10.1111/tra.12812. Traffic. 2021. PMID: 34431177
-
Epidermal growth factor receptor endocytic traffic perturbation by phosphatidate phosphohydrolase inhibition: new strategy against cancer.FEBS J. 2014 May;281(9):2172-89. doi: 10.1111/febs.12770. Epub 2014 Mar 19. FEBS J. 2014. PMID: 24597955
-
Novel mechanism for regulation of epidermal growth factor receptor endocytosis revealed by protein kinase A inhibition.Mol Biol Cell. 2002 May;13(5):1677-93. doi: 10.1091/mbc.01-08-0403. Mol Biol Cell. 2002. PMID: 12006662 Free PMC article.
-
Phospholipase D in endocytosis and endosomal recycling pathways.Biochim Biophys Acta. 2009 Sep;1791(9):845-9. doi: 10.1016/j.bbalip.2009.05.011. Epub 2009 Jun 18. Biochim Biophys Acta. 2009. PMID: 19540357 Free PMC article. Review.
-
New trend in ligand-induced EGFR trafficking: A dual-mode clathrin-mediated endocytosis model.J Proteomics. 2022 Mar 20;255:104503. doi: 10.1016/j.jprot.2022.104503. Epub 2022 Jan 29. J Proteomics. 2022. PMID: 35093568 Review.
Cited by
-
Human rs75776403 polymorphism links differential phenotypic and clinical outcomes to a CLEC18A p.T151M-driven multiomics.J Biomed Sci. 2022 Jun 18;29(1):43. doi: 10.1186/s12929-022-00822-1. J Biomed Sci. 2022. PMID: 35717171 Free PMC article.
-
Phosphatidic acid increases Notch signalling by affecting Sanpodo trafficking during Drosophila sensory organ development.Sci Rep. 2020 Dec 10;10(1):21731. doi: 10.1038/s41598-020-78831-z. Sci Rep. 2020. PMID: 33303974 Free PMC article.
-
Stress-Induced EGFR Trafficking: Mechanisms, Functions, and Therapeutic Implications.Trends Cell Biol. 2016 May;26(5):352-366. doi: 10.1016/j.tcb.2015.12.006. Epub 2016 Jan 27. Trends Cell Biol. 2016. PMID: 26827089 Free PMC article. Review.
-
A kinase inhibitor screen reveals protein kinase C-dependent endocytic recycling of ErbB2 in breast cancer cells.J Biol Chem. 2014 Oct 31;289(44):30443-30458. doi: 10.1074/jbc.M114.608992. Epub 2014 Sep 15. J Biol Chem. 2014. PMID: 25225290 Free PMC article.
-
Sonic Hedgehog modulates EGFR dependent proliferation of neural stem cells during late mouse embryogenesis through EGFR transactivation.Front Cell Neurosci. 2013 Sep 26;7:166. doi: 10.3389/fncel.2013.00166. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24133411 Free PMC article.
References
-
- Bali P. K., Harris W. R. Site-specific rate constants for iron removal from diferric transferrin by nitrilotris(methylenephosphonic acid) and pyrophosphate. Arch. Biochem. Biophys. 1990;281:251–256. - PubMed
-
- Barbier A. J., Poppleton H. M., Yigzaw Y., Mullenix J. B., Wiepz G. J., Bertics P. J., Patel T. B. Transmodulation of epidermal growth factor receptor function by cyclic AMP-dependent protein kinase. J. Biol. Chem. 1999;274:14067–14073. - PubMed
-
- Baron C. L., Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

