Tick-borne encephalitis virus delays interferon induction and hides its double-stranded RNA in intracellular membrane vesicles
- PMID: 20554782
- PMCID: PMC2919015
- DOI: 10.1128/JVI.00176-10
Tick-borne encephalitis virus delays interferon induction and hides its double-stranded RNA in intracellular membrane vesicles
Abstract
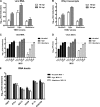
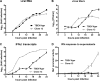
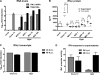
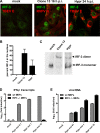
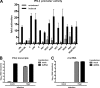
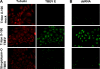
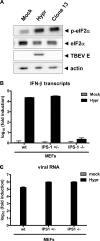
Tick-borne encephalitis virus (TBEV) (family Flaviviridae, genus Flavivirus) accounts for approximately 10,000 annual cases of severe encephalitis in Europe and Asia. Here, we investigated the induction of the antiviral type I interferons (IFNs) (alpha/beta IFN [IFN-alpha/beta]) by TBEV. Using strains Neudörfl, Hypr, and Absettarov, we demonstrate that levels of IFN-beta transcripts and viral RNA are strictly correlated. Moreover, IFN induction by TBEV was dependent on the transcription factor IFN regulatory factor 3 (IRF-3). However, even strain Hypr, which displayed the strongest IFN-inducing activity and the highest RNA levels, substantially delayed the activation of IRF-3. As a consequence, TBEV can keep the level of IFN transcripts below the threshold value that would permit the release of IFN by the cell. Only after 24 h of infection have cells accumulated sufficient IFN transcripts to produce detectable amounts of secreted IFNs. The delay in IFN induction appears not to be caused by a specific viral protein, since the individual expressions of TBEV C, E, NS2A, NS2B, NS3, NS4A, NS4B, NS5, and NS2B-NS3, as well as TBEV infection itself, had no apparent influence on specific IFN-beta induction. We noted, however, that viral double-stranded RNA (dsRNA), an important trigger of the IFN response, is immunodetectable only inside intracellular membrane compartments. Nonetheless, the dependency of IFN induction on IFN promoter stimulator 1 (IPS-1) as well as the phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2alpha) suggest the cytoplasmic exposure of some viral dsRNA late in infection. Using ultrathin-section electron microscopy, we demonstrate that, similar to other flaviviruses, TBEV rearranges intracellular membranes. Virus particles and membrane-connected vesicles (which most likely represent sites of virus RNA synthesis) were observed inside the endoplasmic reticulum. Thus, apparently, TBEV rearranges internal cell membranes to provide a compartment for its dsRNA, which is largely inaccessible for detection by cytoplasmic pathogen receptors. This delays the onset of IFN induction sufficiently to give progeny particle production a head start of approximately 24 h.
Figures
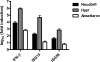

Similar articles
-
Three-dimensional architecture of tick-borne encephalitis virus replication sites and trafficking of the replicated RNA.J Virol. 2013 Jun;87(11):6469-81. doi: 10.1128/JVI.03456-12. Epub 2013 Apr 3. J Virol. 2013. PMID: 23552408 Free PMC article.
-
The stress granule component TIA-1 binds tick-borne encephalitis virus RNA and is recruited to perinuclear sites of viral replication to inhibit viral translation.J Virol. 2014 Jun;88(12):6611-22. doi: 10.1128/JVI.03736-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696465 Free PMC article.
-
Model System for the Formation of Tick-Borne Encephalitis Virus Replication Compartments without Viral RNA Replication.J Virol. 2019 Aug 28;93(18):e00292-19. doi: 10.1128/JVI.00292-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243132 Free PMC article.
-
Steps of the tick-borne encephalitis virus replication cycle that affect neuropathogenesis.Virus Res. 2005 Aug;111(2):161-74. doi: 10.1016/j.virusres.2005.04.007. Virus Res. 2005. PMID: 15871909 Review.
-
Tick-Borne Encephalitis Virus: A Structural View.Viruses. 2018 Jun 28;10(7):350. doi: 10.3390/v10070350. Viruses. 2018. PMID: 29958443 Free PMC article. Review.
Cited by
-
Cellular Lipids-Hijacked Victims of Viruses.Viruses. 2022 Aug 27;14(9):1896. doi: 10.3390/v14091896. Viruses. 2022. PMID: 36146703 Free PMC article. Review.
-
Three-dimensional architecture of tick-borne encephalitis virus replication sites and trafficking of the replicated RNA.J Virol. 2013 Jun;87(11):6469-81. doi: 10.1128/JVI.03456-12. Epub 2013 Apr 3. J Virol. 2013. PMID: 23552408 Free PMC article.
-
Electron Tomography Analysis of Tick-Borne Encephalitis Virus Infection in Human Neurons.Sci Rep. 2015 Jun 15;5:10745. doi: 10.1038/srep10745. Sci Rep. 2015. PMID: 26073783 Free PMC article.
-
In Vivo Characterization of Tick-Borne Encephalitis Virus in Bank Voles (Myodes glareolus).Viruses. 2019 Nov 15;11(11):1069. doi: 10.3390/v11111069. Viruses. 2019. PMID: 31731773 Free PMC article.
-
Tick-Borne Flaviviruses, with a Focus on Powassan Virus.Clin Microbiol Rev. 2018 Dec 12;32(1):e00106-17. doi: 10.1128/CMR.00106-17. Print 2019 Jan. Clin Microbiol Rev. 2018. PMID: 30541872 Free PMC article. Review.
References
-
- Best, S. M., K. L. Morris, J. G. Shannon, S. J. Robertson, D. N. Mitzel, G. S. Park, E. Boer, J. B. Wolfinbarger, and M. E. Bloom. 2005. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 79:12828-12839. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

