Identification of immunologically relevant proteins of Chlamydophila abortus using sera from experimentally infected pregnant ewes
- PMID: 20554807
- PMCID: PMC2916250
- DOI: 10.1128/CVI.00163-10
Identification of immunologically relevant proteins of Chlamydophila abortus using sera from experimentally infected pregnant ewes
Abstract
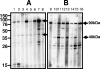
Chlamydophila abortus is an intracellular pathogen and the etiological agent of enzootic abortion of ewes (EAE). C. abortus has a biphasic development cycle; extracellular infectious elementary bodies (EB) attach and penetrate host cells, where they give rise to intracellular, metabolically active reticulate bodies (RB). RB divide by binary fission and subsequently mature to EB, which, on rupture of infected cells, are released to infect new host cells. Pregnant ewes were challenged with 2 x 10(6) inclusion forming units (IFU) of C. abortus cultured in yolk sac (comprising both EB and RB). Serum samples were collected at 0, 7, 14, 21, 27, 30, 35, 40, and 43 days postinfection (dpi) and used to identify antigens of C. abortus expressed during disease. Additionally, sera from fetal lambs were collected at 30, 35, 40, and 43 dpi. All serum samples collected from experimentally infected pregnant ewes reacted specifically with several antigens of EB as determined by one-dimensional (1-D) and 2-D gel electrophoresis; reactive antigens identified by mass spectrometry included the major outer membrane protein (MOMP), polymorphic outer membrane protein (POMP), and macrophage infectivity potentiator (MIP) lipoprotein.
Figures





References
-
- Azuma, Y., H. Hirakawa, A. Yamashita, Y. Cai, M. A. Rahman, H. Suzuki, S. Mitaku, H. Toh, S. Goto, T. Murakami, K. Sugi, H. Hayashi, H. Fukushi, M. Hattori, S. Kuhara, and M. Shirai. 2006. Genome sequence of the cat pathogen, Chlamydophila felis. DNA Res. 13:15-23. - PubMed
-
- Bannantine, J. P., and D. D. Rockey. 1999. Use of a primate model system to identify Chlamydia trachomatis protein antigens recognized uniquely in the context of infection. Microbiology 145:2077-2085. - PubMed
-
- Bas, S., L. Neff, M. Vuillet, U. Spenato, T. Seya, M. Matsumoto, and C. Gabay. 2008. The proinflammatory cytokine response to Chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14. J. Immunol. 180:1158-1168. - PubMed
-
- DAFF. 2006. Regional veterinary laboratories—surveillance report 2006. Department of Agriculture, Fisheries and Food, Dublin, Ireland.
-
- DAFF. 2007. Regional veterinary laboratories—surveillance report 2007. Department of Agriculture, Fisheries and Food, Dublin, Ireland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

