Pretreatment of Guinea pigs with galantamine prevents immediate and delayed effects of soman on inhibitory synaptic transmission in the hippocampus
- PMID: 20554906
- PMCID: PMC2939667
- DOI: 10.1124/jpet.110.167700
Pretreatment of Guinea pigs with galantamine prevents immediate and delayed effects of soman on inhibitory synaptic transmission in the hippocampus
Abstract
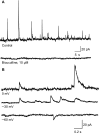
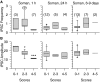
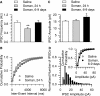
Galantamine has emerged as a potential antidote to prevent the acute toxicity of organophosphorus (OP) compounds. Changes in inhibitory GABAergic activity in different brain regions can contribute to both induction and maintenance of seizures in subjects exposed to the OP nerve agent soman. Here, we tested the hypothesis that galantamine can prevent immediate and delayed effects of soman on hippocampal inhibitory synaptic transmission. Spontaneous inhibitory postsynaptic currents (IPSCs) were recorded from CA1 pyramidal neurons in hippocampal slices obtained at 1 h, 24 h, or 6 to 9 days after the injection of guinea pigs with saline (0.5 ml/kg i.m.), 1xLD(50) soman (26.3 microg/kg s.c.), galantamine (8 mg/kg i.m.), or galantamine at 30 min before soman. Soman-challenged animals that were not pretreated showed mild, moderate, or severe signs of acute intoxication. At 1 h after the soman injection, the mean IPSC amplitude recorded from slices of mildly intoxicated animals and the mean IPSC frequency recorded from slices of severely intoxicated animals were larger and lower, respectively, than those recorded from slices of control animals. Regardless of the severity of the acute toxicity, at 24 h after the soman challenge the mean IPSC frequency was lower than that recorded from slices of control animals. At 6 to 9 days after the challenge, the IPSC frequency had returned to control levels, whereas the mean IPSC amplitude became larger than control. Pretreatment with galantamine prevented soman-induced changes in IPSCs. Counteracting the effects of soman on inhibitory transmission can be an important determinant of the antidotal effectiveness of galantamine.
Figures
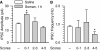


References
-
- Albuquerque EX, Adler M, Pereira EFR. (2005) inventors; University of Maryland, Baltimore, assignee. Method of treating organophosphorous poisoning. International Patent Application PCT/US05/33789. 2005 Sept 23
-
- Albuquerque EX, Deshpande SS, Kawabuchi M, Aracava Y, Idriss M, Rickett DL, Boyne AF. (1985) Multiple actions of anticholinesterase agents on chemosensitive synapses: molecular basis for prophylaxis and treatment of organophosphate poisoning. Fundam Appl Toxicol 5:S182–S203 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

