BCATm deficiency ameliorates endotoxin-induced decrease in muscle protein synthesis and improves survival in septic mice
- PMID: 20554928
- PMCID: PMC2944428
- DOI: 10.1152/ajpregu.00297.2010
BCATm deficiency ameliorates endotoxin-induced decrease in muscle protein synthesis and improves survival in septic mice
Abstract
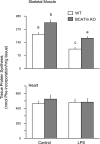
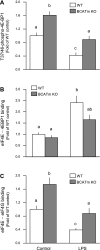
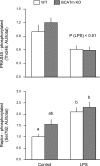
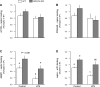
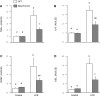
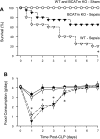
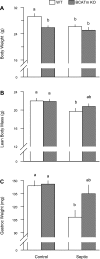
Endotoxin (LPS) and sepsis decrease mammalian target of rapamycin (mTOR) activity in skeletal muscle, thereby reducing protein synthesis. Our study tests the hypothesis that inhibition of branched-chain amino acid (BCAA) catabolism, which elevates circulating BCAA and stimulates mTOR, will blunt the LPS-induced decrease in muscle protein synthesis. Wild-type (WT) and mitochondrial branched-chain aminotransferase (BCATm) knockout mice were studied 4 h after Escherichia coli LPS or saline. Basal skeletal muscle protein synthesis was increased in knockout mice compared with WT, and this change was associated with increased eukaryotic initiation factor (eIF)-4E binding protein-1 (4E-BP1) phosphorylation, eIF4E.eIF4G binding, 4E-BP1.raptor binding, and eIF3.raptor binding without a change in the mTOR.raptor complex in muscle. LPS decreased muscle protein synthesis in WT mice, a change associated with decreased 4E-BP1 phosphorylation as well as decreased formation of eIF4E.eIF4G, 4E-BP1.raptor, and eIF3.raptor complexes. In BCATm knockout mice given LPS, muscle protein synthesis only decreased to values found in vehicle-treated WT control mice, and this ameliorated LPS effect was associated with a coordinate increase in 4E-BP1.raptor, eIF3.raptor, and 4E-BP1 phosphorylation. Additionally, the LPS-induced increase in muscle cytokines was blunted in BCATm knockout mice, compared with WT animals. In a separate study, 7-day survival and muscle mass were increased in BCATm knockout vs. WT mice after polymicrobial peritonitis. These data suggest that elevating blood BCAA is sufficient to ameliorate the catabolic effect of LPS on skeletal muscle protein synthesis via alterations in protein-protein interactions within mTOR complex-1, and this may provide a survival advantage in response to bacterial infection.
Figures
References
-
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130: 2413–2419, 2000 - PubMed
-
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 - PubMed
-
- Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4: 854–865, 2005 - PubMed
-
- De Bandt JP, Cynober L. Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis. J Nutr 136: 308S–313S, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

