Taking the thrombin "fork"
- PMID: 20554951
- PMCID: PMC2888492
- DOI: 10.1161/ATVBAHA.108.179598
Taking the thrombin "fork"
Abstract
The proverb that probably best exemplifies my career in research is attributable to Yogi Berra (http://www.yogiberra.com/), ie, "when you come to a fork in the road ... take it." My career is a consequence of chance interactions with great mentors and talented students and the opportunities provided by a succession of ground-breaking improvements in technology.
Figures

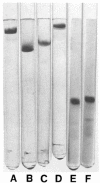

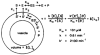




Similar articles
-
The discovery of the endothelial cell protein C receptor.J Thromb Haemost. 2010 Jan;8(1):2-5. doi: 10.1111/j.1538-7836.2009.03660.x. Epub 2009 Oct 23. J Thromb Haemost. 2010. PMID: 19874460 No abstract available.
-
Insight Into the Editor: Nigel Mackman, PhD.Arterioscler Thromb Vasc Biol. 2017 Nov;37(11):e172-e173. doi: 10.1161/ATVBAHA.117.309780. Arterioscler Thromb Vasc Biol. 2017. PMID: 29070540 No abstract available.
-
George J. Broze Jr., MD (3 August 1946-19 June 2019).J Thromb Haemost. 2019 Oct;17(10):1779-1780. doi: 10.1111/jth.14607. J Thromb Haemost. 2019. PMID: 31571416 No abstract available.
-
The conversion of fibrinogen to fibrin: A brief history of some key events.Matrix Biol. 2017 Jul;60-61:5-7. doi: 10.1016/j.matbio.2016.08.002. Epub 2016 Aug 9. Matrix Biol. 2017. PMID: 27519977 Review.
-
Coagulation history, Oxford 1951-53.Br J Haematol. 1999 Oct;107(1):22-32. doi: 10.1046/j.1365-2141.1999.01689.x. Br J Haematol. 1999. PMID: 10520022 Review. No abstract available.
Cited by
-
Thrombin: An Approach to Developing a Higher-Order Reference Material and Reference Measurement Procedure for Substance Identity, Amount, and Biological Activities.J Res Natl Inst Stand Technol. 2020 Jul 29;125:125021. doi: 10.6028/jres.125.021. eCollection 2020. J Res Natl Inst Stand Technol. 2020. PMID: 39035347 Free PMC article. No abstract available.
References
-
- Fish WW, Mann KG, Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969;244:4989–4994. - PubMed
-
- Mann KG, Batt CW. The molecular weights of bovine thrombin and its primary autolysis products. J Biol Chem. 1969;244:6555–6557. - PubMed
-
- Mann KG, Yip R, Heldebrant CM, Fass DN. Multiple active forms of thrombin. 3. Polypeptide chain location of active site serine and carbohydrate. J Biol Chem. 1973;248:1868–1875. - PubMed
-
- Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. - PubMed
-
- Davie EW, Ratnoff OD. Waterfall sequence for intrinstic blood clotting. Science. 1964;145:1310–1312. - PubMed
Publication types
MeSH terms
Substances
Personal name as subject
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

