Human FANCC is hypomorphic in murine Fancc-deficient cells
- PMID: 20554974
- PMCID: PMC2951854
- DOI: 10.1182/blood-2010-02-266411
Human FANCC is hypomorphic in murine Fancc-deficient cells
Abstract
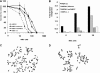
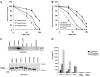
Fancc suppresses cross-linker-induced genotoxicity, modulates growth-inhibitory cytokine responses, and modulates endotoxin responses. Although loss of the latter function is known to account for endotoxin-induced marrow failure in murine Fancc (mFancc)-deficient mice, some argue that cytokine and endotoxin hypersensitivities devolve simply from genomic instability. Seeking to resolve this question, we planned to ectopically express instructive human FANCC (hFANCC) mutants in murine Fancc-deficient hematopoietic stem cells. To first assure that hFANCC cDNA was competent in murine cells, we compared hFANCC and mFancc in complementation assays for cross-linking agent hypersensitivity and endotoxin hypersensitivity. We found that mFancc complemented murine Fancc-deficient cells in both assays, but that hFANCC fully suppressed only endotoxin hypersensitivity, not cross-linking agent hypersensitivity. These results support the notions that Fancc is multifunctional and that structural prerequisites for its genoprotective functions differ from those required to constrain endotoxin responses known to lead to marrow failure in Fancc-deficient mice.
Figures
References
-
- de Winter JP, Joenje H. The genetic and molecular basis of Fanconi anemia. Mutat Res. 2009;668(1):11–19. - PubMed
-
- Vaz F, Hanenberg H, Schuster B, et al. Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat Genet. 201;42(5):406–409. - PubMed
-
- Pang Q, Christianson TA, Keeble W, et al. The Fanconi anemia complementation group C gene product: structural evidence of multifunctionality. Blood. 2001;98(5):1392–1401. - PubMed
-
- Bagby GC, Alter BP. Fanconi anemia. Semin Hematol. 2006;43(3):147–156. - PubMed
-
- Chen M, Tomkins DJ, Auerbach W, et al. Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconi anaemia. Nat Genet. 1996;12(4):448–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

