The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical
- PMID: 20555031
- PMCID: PMC2940533
- DOI: 10.1210/en.2010-0055
The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical
Abstract
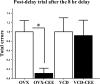
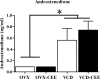
The question of whether to take hormone therapy (HT) will impact every woman as she enters reproductive senescence. In women, studies suggest that ovarian hormone loss associated with menopause has deleterious cognitive effects. Results from clinical studies evaluating whether estrogen-containing HT mitigates these effects, and benefits cognition, are discrepant. Type of menopause, surgical vs. transitional, impacts cognitive outcome in women. However, whether type of menopause impacts cognitive effects of HT has not been methodically tested in women or an animal model. We used the 4-vinylcyclohexene diepoxide rodent model of ovarian follicle depletion, which mimics transitional menopause, and the traditional rat model of menopause, ovariectomy, to cognitively test the most commonly prescribed estrogen therapy in the United States, conjugated equine estrogens (Premarin). Here we show conjugated equine estrogens benefited cognition in surgically menopausal rats, but, in contrast, impaired cognition in transitionally menopausal rats. Androstenedione, released from the residual transitional menopausal ovary, was positively associated with impaired performance, replicating our previous findings in 4-vinylcyclohexene diepoxide animals. The current findings are especially salient given that no clinical study testing cognition has methodically separated these two populations of menopausal women for analysis. That we now show surgical vs. transitional modes of menopause result in disparate cognitive effects of HT has implications for future research and treatments optimizing HT for menopausal women.
Figures

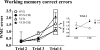


References
-
- Stefanick ML 2005 Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the U.S. Food and Drug Administration. Am J Med 118(Suppl 12B):64–73 - PubMed
-
- Campbell S, Whitehead M 1977 Oestrogen therapy and the menopausal syndrome. Clin Obstet Gynaecol 4:31–47 - PubMed
-
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N 1995 Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia 6:99–107 - PubMed
-
- Kantor HI, Michael CM, Shore H 1973 Estrogen for older women. Am J Obstet Gynecol 116:115–118 - PubMed
-
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones 3rd BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J 2003 Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

