GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice
- PMID: 20555144
- PMCID: PMC5588158
- DOI: 10.3233/JAD-2010-091471
GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice
Abstract
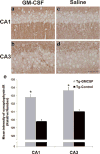
Rheumatoid arthritis (RA) is a negative risk factor for the development of Alzheimer's disease (AD). While it has been commonly assumed that RA patients' usage of non-steroidal anti-inflammatory drugs (NSAIDs) helped prevent onset and progression of AD, NSAID clinical trials have proven unsuccessful in AD patients. To determine whether intrinsic factors within RA pathogenesis itself may underlie RA's protective effect, we investigated the activity of colony-stimulating factors, upregulated in RA, on the pathology and behavior of transgenic AD mice. 5 microg bolus injections of macrophage, granulocyte, and granulocyte-macrophage colony-stimulating factors (M-CSF, G-CSF, or GM-CSF) were administered unilaterally into the hippocampus of aged cognitively-impaired AD mice and the resulting amyloid load reductions determined one week later, using the artificial cerebrospinal fluid-injected contralateral sides as controls. G-CSF and more significantly, GM-CSF reduced amyloidosis throughout the treated brain hemisphere one week following bolus administration to AD mice. 20 daily subcutaneous injections of 5 microg of GM-CSF (the most amyloid-reducing CSF in the bolus experiment) were administered to balanced cohorts of AD mice after assessment in a battery of cognitive tests. Reductions in amyloid load and improvements in cognitive function were assessed. Subcutaneous GM-CSF administration significantly reduced brain amyloidosis and completely reversed the cognitive impairment, while increasing hippocampal synaptic area and microglial density. These findings, along with two decades of accrued safety data using Leukine, recombinant human GMCSF, in elderly leukopenic patients, suggest that Leukine should be tested as a treatment to reverse cerebral amyloid pathology and cognitive impairment in AD.
Figures



Similar articles
-
Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer's mice.Neuroscience. 2009 Sep 29;163(1):55-72. doi: 10.1016/j.neuroscience.2009.05.071. Epub 2009 Jun 14. Neuroscience. 2009. PMID: 19500657 Free PMC article.
-
The innate immune system stimulating cytokine GM-CSF improves learning/memory and interneuron and astrocyte brain pathology in Dp16 Down syndrome mice and improves learning/memory in wild-type mice.Neurobiol Dis. 2022 Jun 15;168:105694. doi: 10.1016/j.nbd.2022.105694. Epub 2022 Mar 18. Neurobiol Dis. 2022. PMID: 35307513 Free PMC article.
-
Granulocyte-macrophage colony-stimulating factor neuroprotective activities in Alzheimer's disease mice.J Neuroimmunol. 2018 Jun 15;319:80-92. doi: 10.1016/j.jneuroim.2018.03.009. Epub 2018 Mar 17. J Neuroimmunol. 2018. PMID: 29573847 Free PMC article.
-
G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis.Nat Rev Rheumatol. 2009 Oct;5(10):554-9. doi: 10.1038/nrrheum.2009.178. Nat Rev Rheumatol. 2009. PMID: 19798030 Review.
-
Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects.Drugs. 2019 Nov;79(16):1741-1755. doi: 10.1007/s40265-019-01192-z. Drugs. 2019. PMID: 31486005 Review.
Cited by
-
Rheumatoid arthritis is a protective factor against Alzheimer's disease: a bidirectional two-sample Mendelian randomization study.Inflammopharmacology. 2024 Feb;32(1):863-871. doi: 10.1007/s10787-023-01397-5. Epub 2023 Dec 27. Inflammopharmacology. 2024. PMID: 38151584
-
A Trisomy 21-linked Hematopoietic Gene Variant in Microglia Confers Resilience in Human iPSC Models of Alzheimer's Disease.bioRxiv [Preprint]. 2024 Mar 14:2024.03.12.584646. doi: 10.1101/2024.03.12.584646. bioRxiv. 2024. PMID: 38559257 Free PMC article. Preprint.
-
Reparative Effects of Stem Cell Factor and Granulocyte Colony-Stimulating Factor in Aged APP/PS1 Mice.Aging Dis. 2020 Dec 1;11(6):1423-1443. doi: 10.14336/AD.2020.0201. eCollection 2020 Dec. Aging Dis. 2020. PMID: 33269098 Free PMC article.
-
Granulocyte-macrophage colony-stimulating factor reduces lung bacterial load following traumatic brain injury and hemorrhage polytrauma in a juvenile rat model.PLoS One. 2025 May 19;20(5):e0323674. doi: 10.1371/journal.pone.0323674. eCollection 2025. PLoS One. 2025. PMID: 40388470 Free PMC article.
-
Single-Cell RNA Sequencing Reveals Immunomodulatory Effects of Stem Cell Factor and Granulocyte Colony-Stimulating Factor Treatment in the Brains of Aged APP/PS1 Mice.Biomolecules. 2024 Jul 10;14(7):827. doi: 10.3390/biom14070827. Biomolecules. 2024. PMID: 39062541 Free PMC article.
References
-
- McGeer PL, Rogers J, McGeer EG. Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. J Alzheimers Dis. 2006;9:271–276. - PubMed
-
- Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. - PMC - PubMed
-
- Okazaki H, Reagan TJ, Campbell RJ. Clinicopathologic studies of primary cerebral amyloid angiopathy. Mayo Clin Proc. 1979;54:22–31. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

