aHUS caused by complement dysregulation: new therapies on the horizon
- PMID: 20556434
- PMCID: PMC2991208
- DOI: 10.1007/s00467-010-1556-4
aHUS caused by complement dysregulation: new therapies on the horizon
Erratum in
- Pediatr Nephrol. 2013 Jan;28(1):165
Abstract
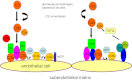
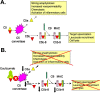
Atypical hemolytic uremic syndrome (aHUS) is a heterogeneous disease that is caused by defective complement regulation in over 50% of cases. Mutations have been identified in genes encoding both complement regulators [complement factor H (CFH), complement factor I (CFI), complement factor H-related proteins (CFHR), and membrane cofactor protein (MCP)], as well as complement activators [complement factor B (CFB) and C3]. More recently, mutations have also been identified in thrombomodulin (THBD), an anticoagulant glycoprotein that plays a role in the inactivation of C3a and C5a. Inhibitory autoantibodies to CFH account for an additional 5-10% of cases and can occur in isolation or in association with mutations in CFH, CFI, CFHR 1, 3, 4, and MCP. Plasma therapies are considered the mainstay of therapy in aHUS secondary to defective complement regulation and may be administered as plasma infusions or plasma exchange. However, in certain cases, despite initiation of plasma therapy, renal function continues to deteriorate with progression to end-stage renal disease and renal transplantation. Recently, eculizumab, a humanized monoclonal antibody against C5, has been described as an effective therapeutic strategy in the management of refractory aHUS that has failed to respond to plasma therapy. Clinical trials are now underway to further evaluate the efficacy of eculizumab in the management of both plasma-sensitive and plasma-resistant aHUS.
Figures
Comment in
-
Long-term renal function under plasma exchange in atypical hemolytic uremic syndrome.Pediatr Nephrol. 2011 Oct;26(10):1915-6. doi: 10.1007/s00467-011-1925-7. Epub 2011 Jun 7. Pediatr Nephrol. 2011. PMID: 21647646 Free PMC article. No abstract available.
Similar articles
-
[Atypical hemolytic-uremic syndrome related to abnormalities within the complement system].Rev Med Interne. 2011 Apr;32(4):232-40. doi: 10.1016/j.revmed.2009.09.039. Epub 2011 Mar 3. Rev Med Interne. 2011. PMID: 21376430 French.
-
Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype.Clin J Am Soc Nephrol. 2010 Oct;5(10):1844-59. doi: 10.2215/CJN.02210310. Epub 2010 Jul 1. Clin J Am Soc Nephrol. 2010. PMID: 20595690 Free PMC article.
-
Transplantation in atypical hemolytic uremic syndrome.Semin Thromb Hemost. 2010 Sep;36(6):653-9. doi: 10.1055/s-0030-1262887. Epub 2010 Sep 23. Semin Thromb Hemost. 2010. PMID: 20865642 Review.
-
Atypical hemolytic uremic syndrome.Orphanet J Rare Dis. 2011 Sep 8;6:60. doi: 10.1186/1750-1172-6-60. Orphanet J Rare Dis. 2011. PMID: 21902819 Free PMC article. Review.
-
Tailored eculizumab therapy in the management of complement factor H-mediated atypical hemolytic uremic syndrome in an adult kidney transplant recipient: a case report.Transplant Proc. 2012 Dec;44(10):3037-40. doi: 10.1016/j.transproceed.2012.07.141. Transplant Proc. 2012. PMID: 23195022
Cited by
-
Pharmacological Management of Atypical Hemolytic Uremic Syndrome in Pediatric Patients: Current and Future.Paediatr Drugs. 2023 Mar;25(2):193-202. doi: 10.1007/s40272-022-00555-6. Epub 2023 Jan 13. Paediatr Drugs. 2023. PMID: 36637720 Free PMC article.
-
Eculizumab in the treatment of atypical hemolytic uremic syndrome in an infant leads to cessation of peritoneal dialysis and improvement of severe hypertension.Pediatr Nephrol. 2015 Apr;30(4):603-8. doi: 10.1007/s00467-014-2975-4. Epub 2014 Oct 16. Pediatr Nephrol. 2015. PMID: 25318620
-
Atypical hemolytic uremic syndrome: a clinical conundrum.Pediatr Nephrol. 2016 Oct;31(10):1615-24. doi: 10.1007/s00467-016-3369-6. Epub 2016 May 2. Pediatr Nephrol. 2016. PMID: 27139899
-
Atypical hemolytic uremic syndrome: when pregnancy leads to lifelong dialysis: a case report and literature review.Cardiovasc Endocrinol Metab. 2021 Mar 17;10(4):225-230. doi: 10.1097/XCE.0000000000000247. eCollection 2021 Dec. Cardiovasc Endocrinol Metab. 2021. PMID: 34765894 Free PMC article.
-
Eculizumab precision-dosing algorithm for thrombotic microangiopathy in children and young adults undergoing HSCT.Blood Adv. 2022 Mar 8;6(5):1454-1463. doi: 10.1182/bloodadvances.2021006523. Blood Adv. 2022. PMID: 35008105 Free PMC article.
References
-
- Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, Rizzoni G, Taylor CM, Kar N, Zimmerhackl LB. A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int. 2006;70:423–431. - PubMed
-
- Karmali MA, Petric M, Lim C, Fleming PC, Steele BT. Escherichia coli cytotoxin, haemolytic-uraemic syndrome, and haemorrhagic colitis. Lancet. 1983;2:1299–1300. - PubMed
-
- Ariceta G, Besbas N, Johnson S, Karpman D, Landau D, Licht C, Loirat C, Pecoraro C, Taylor CM, Kar N, Vandewalle J, Zimmerhackl LB. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol. 2009;24:687–696. - PubMed
-
- Noris M, Remuzzi G. Genetic abnormalities of complement regulators in hemolytic uremic syndrome: how do they affect patient management? Nat Clin Pract Nephrol. 2005;1:2–3. - PubMed
-
- Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

