The interrelationship between mitochondrial dysfunction and transcriptional dysregulation in Huntington disease
- PMID: 20556492
- PMCID: PMC2913874
- DOI: 10.1007/s10863-010-9286-7
The interrelationship between mitochondrial dysfunction and transcriptional dysregulation in Huntington disease
Abstract
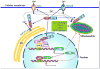
Huntington disease (HD) is an inherited neurodegenerative disease caused by an abnormal expansion of the CAG repeat region in the huntingtin (Htt) gene. Although the pathogenic mechanisms by which mutant Htt (mHtt) causes HD have not been fully elucidated, it is becoming increasingly apparent that mHtt can impair mitochondrial function directly, as well as indirectly by dysregulation of transcriptional processes. mHtt causes increased sensitivity to Ca(2+)-induced decreases in state 3 respiration and mitochondrial permeability transition pore (mPTP) opening concurrent with a reduction in mitochondrial Ca(2+) uptake capacity. Treatment of striatal cells expressing mHtt with thapsigargin results in a decrease in mitochondrial Ca(2+) uptake and membrane potential and an increase in reactive oxygen species (ROS) production. Transcriptional processes regulated by peroxisome proliferator-activated receptor gamma (PPAR gamma) coactivator-1 alpha (PGC-1 alpha), which are critical for mitochondrial biogenesis, have been shown to be impaired in HD. In addition, the PPAR gamma signaling pathway is impaired by mHtt and the activation of this pathway ameliorates many of the mitochondrial deficits, suggesting that PPAR gamma agonists may represent an important treatment strategy for HD.
Figures
References
-
- Arenas J, Campos Y, Ribacoba R, Martin MA, Rubio JC, Ablanedo P, Cabello A. Complex I defect in muscle from patients with Huntington’s disease. Ann Neurol. 1998;43(3):397–400. - PubMed
-
- Beal MF, Brouillet E, Jenkins B, Henshaw R, Rosen B, Hyman BT. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J Neurochem. 1993;61(3):1147–1150. - PubMed
-
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem. 2002;82(3):615–624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

