Co-chaperones are limiting in a depleted chaperone network
- PMID: 20556630
- PMCID: PMC2981734
- DOI: 10.1007/s00018-010-0430-7
Co-chaperones are limiting in a depleted chaperone network
Abstract
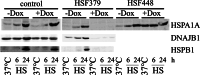
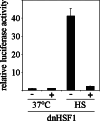
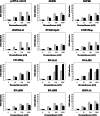
To probe the limiting nodes in the chaperoning network which maintains cellular proteostasis, we expressed a dominant negative mutant of heat shock factor 1 (dnHSF1), the regulator of the cytoplasmic proteotoxic stress response. Microarray analysis of non-stressed dnHSF1 cells showed a two- or more fold decrease in the transcript level of 10 genes, amongst which are the (co-)chaperone genes HSP90AA1, HSPA6, DNAJB1 and HSPB1. Glucocorticoid signaling, which requires the Hsp70 and the Hsp90 folding machines, was severely impaired by dnHSF1, but fully rescued by expression of DNAJA1 or DNAJB1, and partially by ST13. Expression of DNAJB6, DNAJB8, HSPA1A, HSPB1, HSPB8, or STIP1 had no effect while HSP90AA1 even inhibited. PTGES3 (p23) inhibited only in control cells. Our results suggest that the DNAJ co-chaperones in particular become limiting in a depleted chaperoning network. Our results also suggest a difference between the transcriptomes of cells lacking HSF1 and cells expressing dnHSF1.
Figures





Similar articles
-
Protein refolding in peroxisomes is dependent upon an HSF1-regulated function.Cell Stress Chaperones. 2012 Sep;17(5):603-13. doi: 10.1007/s12192-012-0335-5. Epub 2012 Apr 5. Cell Stress Chaperones. 2012. PMID: 22477622 Free PMC article.
-
HtpG Is a Metal-Dependent Chaperone Which Assists the DnaK/DnaJ/GrpE Chaperone System of Mycobacterium tuberculosis via Direct Association with DnaJ2.Microbiol Spectr. 2023 Jun 15;11(3):e0031223. doi: 10.1128/spectrum.00312-23. Epub 2023 Apr 6. Microbiol Spectr. 2023. PMID: 37022172 Free PMC article.
-
Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level.Proc Natl Acad Sci U S A. 2015 May 12;112(19):E2497-506. doi: 10.1073/pnas.1412651112. Epub 2015 Apr 27. Proc Natl Acad Sci U S A. 2015. PMID: 25918398 Free PMC article.
-
Chaperoning signaling pathways: molecular chaperones as stress-sensing 'heat shock' proteins.J Cell Sci. 2002 Jul 15;115(Pt 14):2809-16. doi: 10.1242/jcs.115.14.2809. J Cell Sci. 2002. PMID: 12082142 Review.
-
Neuromuscular Diseases Due to Chaperone Mutations: A Review and Some New Results.Int J Mol Sci. 2020 Feb 19;21(4):1409. doi: 10.3390/ijms21041409. Int J Mol Sci. 2020. PMID: 32093037 Free PMC article. Review.
Cited by
-
Remote Control of Mammalian Cells with Heat-Triggered Gene Switches and Photothermal Pulse Trains.ACS Synth Biol. 2018 Apr 20;7(4):1167-1173. doi: 10.1021/acssynbio.7b00455. Epub 2018 Apr 10. ACS Synth Biol. 2018. PMID: 29579381 Free PMC article.
-
Model systems of protein-misfolding diseases reveal chaperone modifiers of proteotoxicity.Dis Model Mech. 2016 Aug 1;9(8):823-38. doi: 10.1242/dmm.024703. Dis Model Mech. 2016. PMID: 27491084 Free PMC article. Review.
-
Tumor suppressive microRNA-193b promotes breast cancer progression via targeting DNAJC13 and RAB22A.Int J Clin Exp Pathol. 2014 Oct 15;7(11):7563-70. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25550792 Free PMC article.
-
A delayed antioxidant response in heat-stressed cells expressing a non-DNA binding HSF1 mutant.Cell Stress Chaperones. 2013 Jul;18(4):455-73. doi: 10.1007/s12192-012-0400-0. Epub 2013 Jan 16. Cell Stress Chaperones. 2013. PMID: 23321918 Free PMC article.
-
Emerging roles and underlying molecular mechanisms of DNAJB6 in cancer.Oncotarget. 2016 Aug 16;7(33):53984-53996. doi: 10.18632/oncotarget.9803. Oncotarget. 2016. PMID: 27276715 Free PMC article. Review.
References
-
- Kregel KC. Molecular biology of thermoregulation: invited review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. - PubMed
-
- Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–S109. doi: 10.1212/01.wnl.0000192306.98198.ec. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

