Systems microscopy approaches to understand cancer cell migration and metastasis
- PMID: 20556632
- PMCID: PMC2933849
- DOI: 10.1007/s00018-010-0419-2
Systems microscopy approaches to understand cancer cell migration and metastasis
Abstract
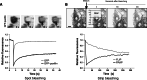
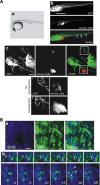
Cell migration is essential in a number of processes, including wound healing, angiogenesis and cancer metastasis. Especially, invasion of cancer cells in the surrounding tissue is a crucial step that requires increased cell motility. Cell migration is a well-orchestrated process that involves the continuous formation and disassembly of matrix adhesions. Those structural anchor points interact with the extra-cellular matrix and also participate in adhesion-dependent signalling. Although these processes are essential for cancer metastasis, little is known about the molecular mechanisms that regulate adhesion dynamics during tumour cell migration. In this review, we provide an overview of recent advanced imaging strategies together with quantitative image analysis that can be implemented to understand the dynamics of matrix adhesions and its molecular components in relation to tumour cell migration. This dynamic cell imaging together with multiparametric image analysis will help in understanding the molecular mechanisms that define cancer cell migration.
Figures





References
-
- Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

